PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1273411

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1273411
NGS Sample Preparation Market - Growth, Trends, and Forecasts (2023 - 2028)
The NGS sample preparation market is expected to register a CAGR of 12.4% over the forecast period.
The outbreak of the COVID-19 pandemic has impacted the NGS sample preparation market. The increasing coronavirus cases worldwide led to the need to develop effective and quick sequencing technologies to rebuild the genomic sequence of SARS-CoV-2, which was the etiological agent of COVID-19. It was critical in the development of diagnostic molecular tests as well as the development of efficient tactics and strategies to slow the spread of the pandemic. For instance, in August 2020, Helix Laboratory received the U.S. FDA emergency use authorization for NGS based COVID-19 test, which is designed to detect the SARS-CoV-2 spike protein gene in upper respiratory specimens. This next-generation sequencing was applied
for the study of COVID-19 and has greatly promoted SARS-CoV-2 original tracking. Since next-generation sequencing (NGS) sample preparation played a vital role during the pandemic, thus the market witnessed positive growth and is expected to continue the upward trend over the forecast period.
The rising infectious disease prevalence reduced sequencing cost, and technical advancement in NGS platforms are the major factors propelling the market growth. With the increasing prevalence of infectious diseases, NGS sequencing technologies have swiftly become the method of choice in virology for a wide range of applications, including the detection of novel viruses from metagenomic samples, reconstruction of whole or almost complete viral genome sequences, and viral evolution and subspecies analysis. For instance, as per the WHO August 2022 Influenza Update, National Influenza Centers (NICs) and other national influenza laboratories from 120 countries, regions, or territories submitted data to FluNet from 11 July 2022 to 24 July 2022. Over 145,086 samples were analyzed by the WHO Global Influenza Surveillance and Response System (GISRS) laboratories during that time. Among the 6,449 people who tested positive for influenza viruses, 97.7% had influenza A, and 2.3% had influenza B.
Similarly, according to the WHO report 2021, each year, typhoid fever is estimated to affect 21 million people worldwide. Similarly, according to the CDC, 30,000 cases of yellow fever occur annually, out of which 90.0% occur in Africa. Therefore, increasing cases of infectious diseases across the globe is anticipated to propel market growth over the forecast period.
The key and small players' launch of new NGS technologies drive market growth. NGS technologies have replaced traditional sequencing methods because of the low cost and high sequencing efficiency. For instance, according to Illumina, in 2021, the cost of next-generation sequencing (NGS) has decreased dramatically since the completion of the Human Genome Project. Illumina has helped reduce the cost of NGS, enabling the USD 1000 human genome. Therefore, new product launches are expected to surge the market growth over the forecast period.
However, the high costs of NGS sample preparation equipment and strict regulation associated with NGS Sample preparation are likely to impede the market growth.
NGS Sample Preparation Market Trends
Diagnostics Segment is Expected to Witness Considerable Growth Over the Forecast Period
NGS can sequence hundreds and thousands of genes or whole genomes quickly. The sequence variants/mutations detected by NGS have been widely used for disease diagnosis, prognosis, therapeutic decision, and follow-up of patients.
The research and treatment of cancer have been transformed by next-generation sequencing to a great extent. The NGS of individual cancer-patient genomes is carried out using NGS base genomic sequencing, and it has emerged as one of the fastest and less expensive methods. For instance, in August 2021, the European Society for Medical Oncology (ESMO), the medical oncology professional organization, launched its first recommendations on the use of next-generation sequencing (NGS) for patients with metastatic cancers. According to ESMO recommendations, NGS could be an alternative to Polymerase Chain Reaction (PCR) in colon cancers. NGS can be suitable for identifying multiple genes and their mutations concurrently by sequencing millions of DNA reads and driving therapeutic decision-making. This is projected to increase in adoption of next-generation sequencing sample preparation for drug development.
The advancements in technology, increasing product approvals and launches, partnerships, and collaborations by key players are also driving growth in the diagnostics segment. For instance, in February 2021, QIAGEN and INOVIO expanded their partnership to create a companion diagnostic using next-generation sequencing (NGS) for INOVIO's VGX-3100 for advanced cervical dysplasia. Furthermore, in Aug 2020, Guardant Health Inc. received the United States Food and Drug Administration (FDA) approval for Guardant360 CDx, which uses two combined technology, Liquid biopsy, and NGS, in one diagnostic test. Hence, the increasing product approval related to NGS diagnostic and product launches may create new opportunities for the segment, owing to which considerable segment growth is anticipated over the forecast period.
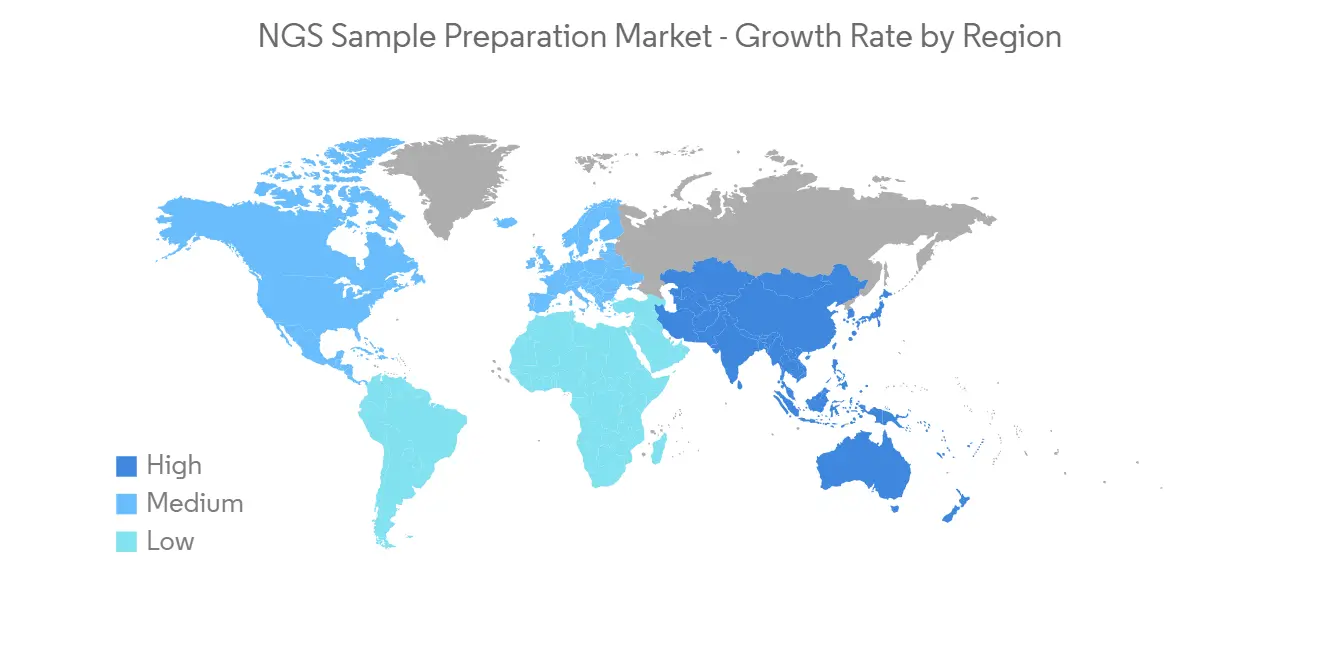
North America is Expected to Witness Significant Growth Over the Forecast Period
North America is expected to witness considerable market growth owing to factors such as the rising need for diagnostics tools for identifying health disparities, and the rising burden of infectious diseases and chronic diseases in the region. The increasing technological advancement, product launches, partnerships, and acquisitions by the key market players are leading to an increase in market growth in North America. For instance, in January 2022, Illumina Inc. and Nashville Biosciences LLC, a subsidiary of Vanderbilt University Medical Center (VUMC), entered a multi-year agreement to accelerate medicines development through large-scale genomics and the establishment of a clinical-genomic resource using Illumina's next-generation sequencing (NGS) platforms. Such initiatives are expected to drive the growth of the NGS sample preparation market in North America.
Key product launches, high concentration of market players or manufacturer's presence, and acquisition & partnerships among major players, and increased funding from the federal government and private players, as well as increased adoption of NGS technology by non-government and government bodies in the United States, are some of the factors driving the growth of the NGS sample preparation market in the country. For instance, in April 2021, Resolution Bioscience, a leader in the research and commercialization of next-generation sequencing (NGS)-based precision oncology solutions, has been acquired by Agilent Technologies. Similarly, in January 2020, in the United States, the Intelligence Advanced Research Projects Activity provided USD 23.0 million to the Broad Institute and Harvard University, and DNA Script. The organizations have been working together to explore the possibility of combining the enzymatic DNA synthesis technology and NGS into a single instrument for more than four years. Therefore, such positive developments are anticipated to boost the market growth in the United States over the forecast period.
NGS Sample Preparation Industry Overview
The NGS sample preparation market is slightly consolidated due to the presence of a few players operating globally and regionally. The competitive landscape includes an analysis of a few international as well as local companies which hold market shares and are well known, including Illumina, Inc., Agilent Technologies, Inc., Bio-Rad Laboratories, Inc., Thermo Fisher Scientific Inc., Qiagen N.V., PerkinElmer Inc., F.Hoffmann-La Roche Ltd, BGI Genomics, DNASTAR, Eurofins Scientific, Danaher Corporation (Beckman Coulter), Macrogen Inc., Integrated DNA Technologies, Inc. (Swift Biosciences Inc.), Genomatix, and Helix OpCo, LLC among others.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Rising Prevalence of Infectious Diseases
- 4.2.2 Reduced Cost of Sequencing
- 4.2.3 Technical Advancement in NGS Platforms
- 4.3 Market Restraints
- 4.3.1 High Costs of NGS Sample Preparation Equipment
- 4.3.2 Strict Regulation Associated with NGS Sample Preparation
- 4.4 Porter's Five Force Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD Million)
- 5.1 By Product Class
- 5.1.1 Reagent and Consumables
- 5.1.2 Workstations
- 5.2 By Applications
- 5.2.1 Diagnostics
- 5.2.2 Drug Discovery
- 5.2.3 Other Applications
- 5.3 End User
- 5.3.1 Hospitals and Clinics
- 5.3.2 Pharmaceutical and Biotechnology Companies
- 5.3.3 Other End Users
- 5.4 Geography
- 5.4.1 North America
- 5.4.1.1 United States
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 Europe
- 5.4.2.1 Germany
- 5.4.2.2 United Kingdom
- 5.4.2.3 France
- 5.4.2.4 Italy
- 5.4.2.5 Spain
- 5.4.2.6 Rest of Europe
- 5.4.3 Asia-Pacific
- 5.4.3.1 China
- 5.4.3.2 Japan
- 5.4.3.3 India
- 5.4.3.4 Australia
- 5.4.3.5 South Korea
- 5.4.3.6 Rest of Asia-Pacific
- 5.4.4 Middle East and Africa
- 5.4.4.1 GCC
- 5.4.4.2 South Africa
- 5.4.4.3 Rest of Middle East and Africa
- 5.4.5 South America
- 5.4.5.1 Brazil
- 5.4.5.2 Argentina
- 5.4.5.3 Rest of South America
- 5.4.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Illumina, Inc.
- 6.1.2 Agilent Technologies, Inc.
- 6.1.3 Bio-Rad Laboratories, Inc.
- 6.1.4 Thermo Fisher Scientific Inc.
- 6.1.5 Qiagen N.V.
- 6.1.6 PerkinElmer Inc.
- 6.1.7 F. Hoffmann-La Roche Ltd
- 6.1.8 BGI Genomics
- 6.1.9 DNASTAR
- 6.1.10 Eurofins Scientific
- 6.1.11 Danaher Corporation (Beckman Coulter)
- 6.1.12 Macrogen Inc.
- 6.1.13 Integrated DNA Technologies, Inc. (Swift Biosciences Inc.)
- 6.1.14 Genomatix
- 6.1.15 Helix OpCo, LLC.
7 MARKET OPPORTUNITIES AND FUTURE TRENDS




