PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1273433

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1273433
Point of Care Diagnostics Market - Growth, Trends, and Forecasts (2023 - 2028)
The point-of-care (POC) diagnostics market size is currently valued at USD 38.03 billion and is expected to register a CAGR of 9.8% during the forecast period.
The COVID-19 pandemic had a significant impact on the growth of the POC diagnostics market. For instance, an article published by the journal Cureus in August 2022 reported that with the emergence of COVID-19, testing became essential for containment and mitigation purposes, and this led to the development of rapid COVID-19 testing. Thus, the COVID-19 pandemic surged the demand for POC diagnostics. However, in the current scenario, due to the ease, affordability, reduced incidence of complications, and faster turn-around times of POC diagnostics, the demand for POC diagnostics is expected to witness significant growth over the forecast period.
The factors that are driving the growth of the studied market are the rising prevalence of chronic and infectious diseases, the increasing number of regulatory approvals for novel immunoassay techniques and technological advancements, and the rising usage of home-based POC devices.
The prevalence of chronic and infectious diseases, such as diabetes, rheumatism, and malaria, is increasing globally, which is propelling the demand for POC diagnostics. For instance, in December 2022, World Health Organization (WHO) reported that malaria is a life-threatening disease, and in the year 2021, there were an estimated 247 million cases of malaria worldwide. Similarly, an article published by NCBI in May 2021, reported that the global prevalence of rheumatoid arthritis (RA) was 460 per 100,000 population during 2020-2021. Thus, a high number of chronic and infectious diseases recorded globally is increasing the demand for rapid diagnostics, thereby increasing the demand for POC diagnostics and thus driving the growth of the studied market.
Moreover, the increasing number of regulatory approvals for novel immunoassay techniques, technological advancements, and the rising usage of home-based POC devices is expected to complement the growth of the studied market over the forecast period. For instance, in July 2022, BioGX launched CE-marked, three-gene multiplex POC COVID-19 test. These tests make use of BioGX's incredibly effective Xfree direct sample testing chemistry, which is present in their Xfree COVID-19 Direct RT-PCR assay, which has been cleared for emergency use by the United States Food and Drug Administration (USFDA). Thus, regulatory approvals for new product launches are contributing to the growth of the studied market.
Furthermore, an article published by Mylab Discovery Solutions in March 2022 reported that POC diagnostics, which are often characterized by being independent of laboratory infrastructure and being highly affordable, can greatly improve the accessibility of diagnostics. The article also quoted that for the people living in low and middle income countries where the healthcare distribution is quite uneven, the use of POC tests is growing as an important tool to increase diagnostic coverage of the population, and thus demand for POC is constantly increasing, thereby driving the growth of the studied market.
Thus, due to the rising prevalence of chronic and infectious diseases, the increasing number of regulatory approvals for novel immunoassay techniques and technological advancements, and the rising usage of home-based POC devices. However, factors such as product recall incidence by the major players in the market, stringent regulatory policies by the governments, and reimbursement issues will impede the growth of the POC diagnostics market during the forecast period.
Point of Care Diagnostics Market Trends
Blood Glucose Testing is Expected to Witness Significant Growth Over the Forecast Period.
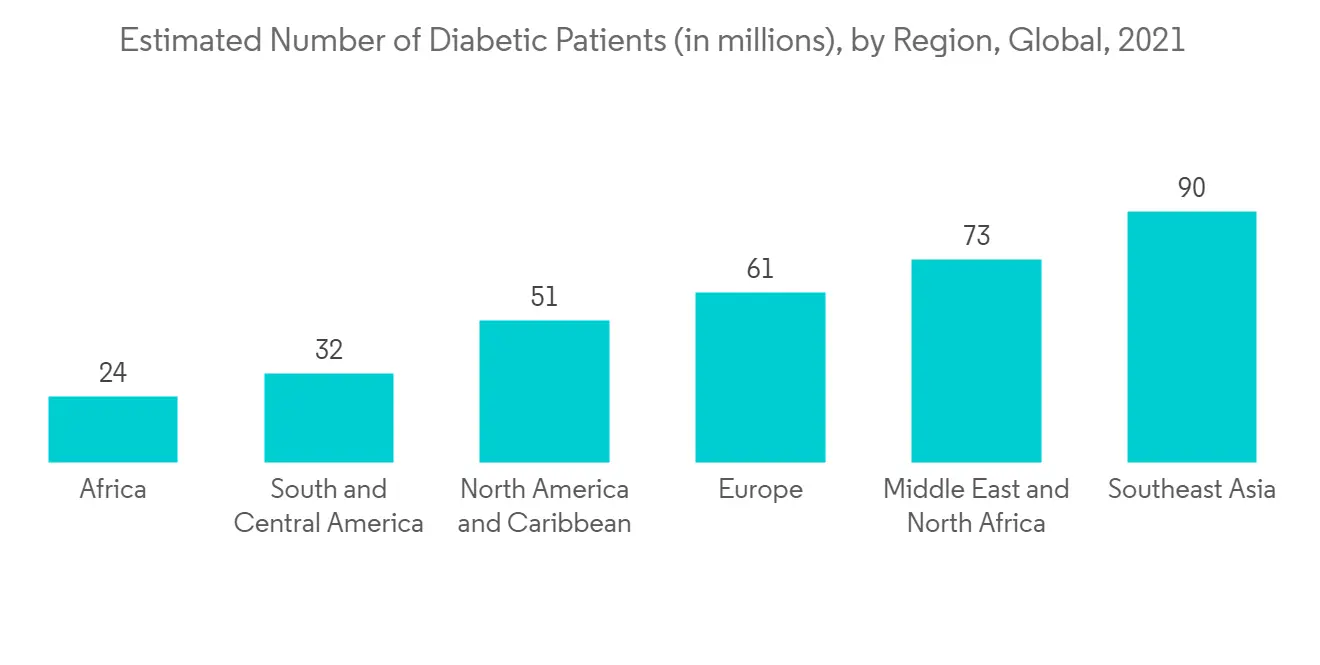
A blood glucose test measures the glucose in the blood, and this is one of the most common tests done across the globe. Blood glucose testing POC diagnostics is expected to witness significant growth over the forecast period owing to rising diabetes cases recorded globally and the new product launches of the company.
The growing prevalence of diabetes and the introduction of portable diagnostic equipment are expected to boost segment growth. For instance, in 2021, International Diabetes Federation (IDF) reported that globally in the year 2021, a total of 536,600.0 per 10000 adults aged between (20-79) years were suffering from diabetes, and this number is expected to increase to 642,800.0 per 1000 adults by 2030 and 783,700.0 per 1000 adults by 2045. Thus, rising diabetes cases are a matter of concern, and there is increasing demand for rapid diagnostics. Thus, rising diabetes cases are increasing the demand for POC diagnostics.
Furthermore, the introduction of point-of-care diagnostics and its increasing use is driving the growth of the studied segment. For instance, in May 2022, LumiraDx, a UK-based healthcare company that manufactures a diagnostic platform to support a menu of tests reported that its HbA1c test received a CE mark for the screening and monitoring of people with diabetes in the point of care (POC) setting. Thus, the launch of such POC is also contributing to the growth of the studied market.
Thus, due to the rising diabetes cases and new product launches, the segment is expected to witness significant growth over the forecast period.
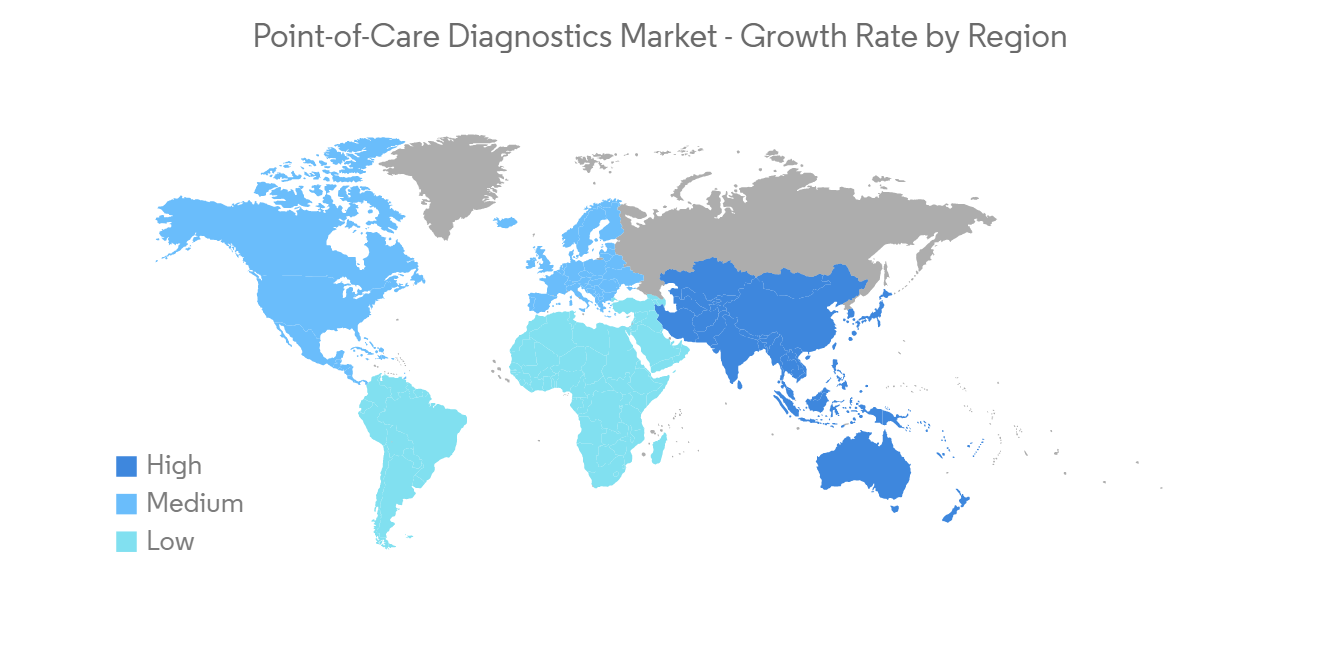
North America is Expected to Witness a Significant Growth Over the Forecast Period.
North America region consists of the United States, Canada, and Mexico. The region is expected to witness significant over the forecast period due to the presence of key market players and the rising incidence of chronic and infectious diseases.
The recent acquisition and product launches are contributing to the growth of the studied market. For instance, in January 2023, Heska Corporation, a US-based company and a global provider of advanced veterinary diagnostic and specialty products and solutions, acquired MBio Diagnostics, Inc. Heska Corporation manufactures, develops, and sells advanced veterinary diagnostic and specialty healthcare products, including POC testing instruments. Thus such acquisitions are driving the growth of the studied market in the region.
Additionally, in May 2022, NOWDiagnostic's ADEXUSDx hCG Test was used for the qualitative detection of human chorionic gonadotropin (hCG) in human whole blood, plasma, or serum received the USFDA clearance, and the test available in the United States. This immunoassay test is an early detection aid for pregnancy for healthcare professionals in a variety of clinical and critical care settings. Thus, such clearance of new POC diagnostics by regulatory agencies is contributing to the studied market's growth in the region.
Moreover, the rising prevalence of diseases, such as diabetes and cancer, in the region is contributing to the market's growth. For instance, as per a report by the Canadian Diabetes Association published in March 2022, diabetes is increasing aggressively in the country, and by the time the report was published, stated that 11.7 million Canadians were living with diabetes or prediabetes, among which is nearly more than 5.7 million Canadians were living with diagnosed diabetes. Such an increasing burden of diabetes is increasing the demand for POC and thus driving the growth of the studied market.
Similarly, in the year 2022, the report of the World Cancer Research Fund International (WCRF) stated that nearly 101,703 cancer cases were diagnosed in Mexico. The POC is also used for the early detection of cancer, and the high number of cancer cases contributes to the growth of the studied market in the region.
Thus, the recent acquisition between the key market players, product launches, and the rising incidence of chronic diseases is expected to drive the growth of the market in the region over the forecast period.
Point of Care Diagnostics Industry Overview
The point-of-care diagnostics market is highly competitive and consists of several major players. In terms of market share, few of the major players currently dominate the market. Some of the major players in the market are Abbott Laboratories, Siemens Healthineers AG, Danaher Corporation (Beckman Coulter Inc.), Becton, Dickinson and Company, Instrumentation Laboratory, Johnson and Johnson Inc., Nova Biomedical Corporation, Qiagen Inc., F. Hoffmann-La Roche Ltd., and Biomeriux SA.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Rising Prevalence of Chronic and Infectious Diseases
- 4.2.2 Increasing Number of Regulatory Approvals for Novel Immunoassay Techniques
- 4.2.3 Technological Advancements and Rising Usage of Home-based POC Devices
- 4.3 Market Restraints
- 4.3.1 Product Recalls
- 4.3.2 Stringent Regulatory Policies and Reimbursement Issues
- 4.4 Porter Five Forces
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD million)
- 5.1 By Product
- 5.1.1 Glucose Monitoring Kit
- 5.1.2 Cardio-metabolic Monitoring Kit
- 5.1.3 Pregnancy and Fertility Testing Kit
- 5.1.4 Infectious Disease Testing Kit
- 5.1.5 Cholesterol Test Strip
- 5.1.6 Hematology Testing Kit
- 5.1.7 Other Products
- 5.2 By End User
- 5.2.1 Hospital and Critical Care Setting
- 5.2.2 Ambulatory Care Setting
- 5.2.3 Research Laboratory
- 5.2.4 Other End Users
- 5.3 Geography
- 5.3.1 North America
- 5.3.1.1 United States
- 5.3.1.2 Canada
- 5.3.1.3 Mexico
- 5.3.2 Europe
- 5.3.2.1 Germany
- 5.3.2.2 United Kingdom
- 5.3.2.3 France
- 5.3.2.4 Italy
- 5.3.2.5 Spain
- 5.3.2.6 Rest of Europe
- 5.3.3 Asia-Pacific
- 5.3.3.1 China
- 5.3.3.2 Japan
- 5.3.3.3 India
- 5.3.3.4 Australia
- 5.3.3.5 South Korea
- 5.3.3.6 Rest of Asia-Pacific
- 5.3.4 Middle East and Africa
- 5.3.4.1 GCC
- 5.3.4.2 South Africa
- 5.3.4.3 Rest of Middle East and Africa
- 5.3.5 South America
- 5.3.5.1 Brazil
- 5.3.5.2 Argentina
- 5.3.5.3 Rest of South America
- 5.3.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Abbott Laboratories
- 6.1.2 Siemens Healthineers AG
- 6.1.3 Danaher Corporation (Beckman Coulter Inc.)
- 6.1.4 Becton, Dickinson and Company
- 6.1.5 Instrumentation Laboratory
- 6.1.6 Johnson and Johnson Inc.
- 6.1.7 Nova Biomedical Corporation
- 6.1.8 Qiagen Inc.
- 6.1.9 F. Hoffmann-La Roche Ltd.
- 6.1.10 Biomeriux SA
7 MARKET OPPORTUNITIES AND FUTURE TRENDS




