Need help finding what you are looking for?
Contact Us
PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1405723

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1405723
Intravenous (IV) Equipment - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts 2024 - 2029
PUBLISHED:
PAGES: 114 Pages
DELIVERY TIME: 2-3 business days
SELECT AN OPTION
The intravenous (IV) equipment market is expected to register a CAGR of 4.3% during the forecast period.
Key Highlights
- The sudden outbreak of COVID-19 had a severe impact on the intravenous (IV) equipment industry due to a sudden halt in surgical procedures that were non-immediate and were being postponed to decrease the burden on healthcare infrastructure. The use of intravenous therapies was restricted for the treatment of COVID-19 to reduce the chances of infection. For instance, as per the guidelines published by the National Institute of Health in July 2023, it was found that the COVID-19 Treatment Guidelines Panel recommended the utilization of IV Immunoglobulin for treating acute COVID-19 infection. Therefore, with the restricted use of IV for COVID-19 treatment, there was a considerable impact on the market studied. However, currently, the market has reached its pre-pandemic nature in terms of demand for IV equipment and is expected to witness strong growth in the coming years.
- Some of the major chronic diseases include cancer, kidney failure, and heart disease, among others, and several lifestyle disorders such as hypertension, diabetes, obesity, and depression, among others, which need critical care during their hospitalization.
- The rise in such chronic diseases is augmenting the growth of the market studied. For instance, according to the World Health Organization data published in April 2021, chronic diseases are expected to kill 41 million people each year, equivalent to 71% of all deaths globally. Also, 77% of mortality in low- and middle-income nations is attributable to chronic diseases. With the increase in the number of cancer cases and other chronic conditions, there will be a growing demand for various surgical procedures, which is believed to propel the market growth.
- Moreover, according to the Alzheimer Society of Canada, in January 2021, over 500,000 Canadians were living with dementia. By 2030, this number is expected to rise to 912,000. Similarly, according to the Fondation de France in 2021, Parkinson's disease affected around 150,000 people in France as of 2021. It also stated that the disease affects the geriatric population more. Hence, the high number of people living with chronic diseases is expected to propel the market growth in the coming years.
- However, stringent regulation and medication errors associated with infusion pumps leading to a product recall are expected to restrain the market growth over the forecast period.
Intravenous (IV) Equipment Market Trends
IV Catheters Segment Holds Significant Share in Intravenous (IV) Equipment Market
- IV catheters are the essential tools to deliver IV medications, blood products, and nutritional fluids to patients. There are different types of IV catheters, namely peripheral IV catheters, central venous catheters, and midline catheters. Healthcare providers administer and use each type of IV catheter for specific treatment purposes.
- Increasing demand for short-closed peripheral IV catheters as they are capable of reducing complications during intravenous procedures is likely to propel the IV catheter segment growth. Additionally, a closed IV catheter system offers protection against the risks associated with needle-stick injury.
- The high incidence of needle-stick injuries is persuading healthcare professionals to use drug delivery devices that reduce such a risk and increase patient comfort. As per the study published by the National Institute of Health in July 2022, approximately 385,000 needle-stick injuries occur in the United States each year. Thus, the high number of needle-stick injuries is promoting the use of safer devices, thereby surging the growth of the IV catheter segment. Moreover, in July 2022, B. Braun Medical Inc. launched its new Introcan Safety 2 IV Catheter with one-time blood control. As the device is automatically controlled, it is considered to be significantly safer than manual catheters. Similarly, in August 2022, a Japanese utility patent was granted to SafeBreak Vascular. The device is mainly used to protect the peripheral IV lines. With this patent, Lineus Medical, a manufacturer of SafeBreak Vascular, entered the Japanese market.
- Thus, all aforementioned factors, such as the rising incidence of needle-stick injuries and increasing product launches, are expected to boost the segment over the forecast period.
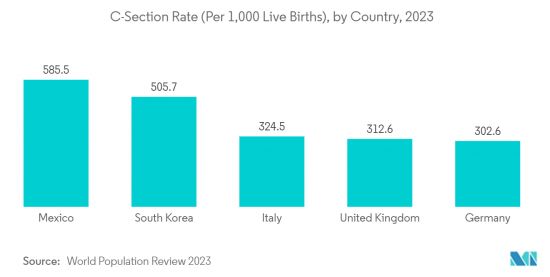
North America is Expected to Dominate the Global Intravenous (IV) Equipment Market
- The growing prevalence of chronic diseases, increasing surgical procedures in the region, and product launches by the major market players are driving the market growth of the North American region.
- According to a study by the National Center for Biotechnology Information published in 2021, about 50 million surgeries are performed in the United States. The rising prevalence of cardiovascular disorders and obesity rates is attributed to the market growth in this region. As per the 'Cardiac Bypass Surgeries' Article published by the University of Alabama in November 2021, nearly 350,000 Coronary Artery Bypass Graft (CABG) Surgeries are done each year in the United States. Such a high number of surgeries is augmenting the demand for intravenous equipment in the country and contributing to market growth.
- Furthermore, approvals from the United States Food and Drug Administration and product launches by key players are expected to boost the market. For instance, in August 2022, Baxter announced the United States Food and Drug Administration (USFDA) clearance of the Novum IQ Syringe infusion pump with dose IQ safety software. The Novum IQ SYR has been designed with enhanced safety features, better connectivity, and accuracy. This software is helpful in providing personalized therapy for patients, including neonates. Similarly, in February 2021, United Therapeutics Corporation launched the Remunity Pump for Remodulin in the United States. The launch was majorly focused on patients suffering from pulmonary atrial hypertension (PAH). Hence, such new launches and approvals across the country are propelling the market growth.
Intravenous (IV) Equipment Industry Overview
The intravenous (IV) equipment market is moderately competitive with the presence of local as well as international companies. Strong competition and rapid technological advancements are key factors fueling the market growth. Companies like Becton, Dickinson and Company, B. Braun, Melsungen AG, 3M, Henry Schein, Inc., and ICU Medical, Inc., among others, hold a substantial market share in the industry.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
Product Code: 68124
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Increasing Prevalence of Chronic Diseases Coupled with Rising Geriatric Population
- 4.2.2 Increasing Number of Surgeries Globally
- 4.3 Market Restraints
- 4.3.1 Medication Errors Associated with Infusion Pumps Leading to Product Recalls
- 4.3.2 Stringent Regulatory Scenario
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD)
- 5.1 By Type
- 5.1.1 IV Catheters
- 5.1.2 Infusion Pumps
- 5.1.3 Securement Devices
- 5.1.4 Administration Sets
- 5.1.5 Drip Chambers
- 5.1.6 Other Tyoes
- 5.2 By End-User
- 5.2.1 Hospitals
- 5.2.2 Ambulatory Surgical Centers
- 5.2.3 Other End-Users
- 5.3 Geography
- 5.3.1 North America
- 5.3.1.1 United States
- 5.3.1.2 Canada
- 5.3.1.3 Mexico
- 5.3.2 Europe
- 5.3.2.1 Germany
- 5.3.2.2 United Kingdom
- 5.3.2.3 France
- 5.3.2.4 Italy
- 5.3.2.5 Spain
- 5.3.2.6 Rest of Europe
- 5.3.3 Asia-Pacific
- 5.3.3.1 China
- 5.3.3.2 Japan
- 5.3.3.3 India
- 5.3.3.4 Australia
- 5.3.3.5 South Korea
- 5.3.3.6 Rest of Asia-Pacific
- 5.3.4 Middle-East and Africa
- 5.3.4.1 GCC
- 5.3.4.2 South Africa
- 5.3.4.3 Rest of Middle-East and Africa
- 5.3.5 South America
- 5.3.5.1 Brazil
- 5.3.5.2 Argentina
- 5.3.5.3 Rest of South America
- 5.3.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 3M
- 6.1.2 Baxter
- 6.1.3 Becton, Dickinson and Company
- 6.1.4 B. Braun Melsungen AG
- 6.1.5 EuroLife Healthcare Pvt. Ltd
- 6.1.6 Henry Schein, Inc
- 6.1.7 ICU Medical, Inc
- 6.1.8 Polymedicure
- 6.1.9 Terumo Corporation
- 6.1.10 Teleflex Incorporated
- 6.1.11 Ascor S.A.
7 MARKET OPPORTUNITIES AND FUTURE TRENDS
Have a question?


SELECT AN OPTION
Have a question?


Questions? Please give us a call or visit the contact form.
