PUBLISHER: Coherent Market Insights | PRODUCT CODE: 1349823

PUBLISHER: Coherent Market Insights | PRODUCT CODE: 1349823
U.S. Implantable Medical Devices Market, By implant type, By End user- Size, Share, Outlook, and Opportunity Analysis, 2023 - 2030.
U.S. Implantable medical devices market is estimated to be valued at US$ 19.27 Bn in 2023 and is expected to exhibit a CAGR of 6.1% during the forecast period (2023-2030).
| Report Coverage | Report Details | ||
|---|---|---|---|
| Base Year: | 2022 | Market Size in 2023: | US$ 19.27 Bn |
| Historical Data for: | 2018 to 2021 | Forecast Period: | 2023 - 2030 |
| Forecast Period 2023 to 2030 CAGR: | 6.10% | 2030 Value Projection: | US$ 29.10 Bn |
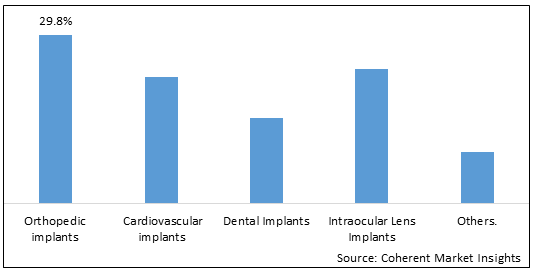
The U.S. implantable medical devices market is a vital component of the healthcare industry, encompassing a wide range of devices that are designed to be implanted in the human body for therapeutic or diagnostic purposes. These devices play a crucial role in improving patient outcomes, enhancing quality of life, and addressing various medical conditions.
Implantable medical devices in the U.S. market include a diverse array of products, such as cardiovascular implants (e.g., pacemakers, stents), orthopaedic implants (e.g., joint replacements, spinal implants), neuro-stimulation devices (e.g., deep brain stimulators, cochlear implants), ophthalmic implants (e.g., intraocular lenses), and many others. These devices are developed using advanced technologies, materials, and manufacturing processes to ensure their safety, efficacy, and long-term reliability.
The U.S. regulatory landscape, overseen by the Food and Drug Administration (FDA), ensured that implantable medical devices meet stringent safety and efficacy standards before market approved. The FDA's rigorous approval process, including pre-market testing, clinical trials, and post-market surveillance, helps ensure patient safety and fosters confidence in the quality and reliability of these devices.
Market Dynamics
Increasing incidence of aging issues afflicted with chronic diseases
The U.S. implantable medical devices market is propelled by a relentless wave of technological advancements, catering to the ever-growing demands of an aging population afflicted with chronic diseases. Stringent regulatory oversight ensures patient safety and product effectiveness, while reimbursement policies and healthcare reforms dictate the market's accessibility and adoption rates. As industry players forge strategic partnerships and engage in mergers and acquisitions, the market undergoes dynamic transformations, driving innovation and shaping the competitive landscape. To thrive in this fast-paced ecosystem, stakeholders must harness these powerful dynamics, capitalizing on emerging opportunities to revolutionize patient care and improve lives.
Key features of the study:
- This report provides in-depth analysis of the U.S. implantable medical devices market and provides market size (US$ Billion) and compound annual growth rate (CAGR %) for the forecast period (2023-2030), considering 2022, as the base year
- It elucidates potential revenue opportunities across different segments and explains attractive investment proposition matrix for this market
- This study also provides key insights about market drivers, restraints, opportunities, new product launches or approval, regional outlook, and competitive strategy adopted by leading players
- It profiles leading players in the U.S. implantable medical devices market based on the following parameters- company overview, financial performance, product portfolio, geographical presence, distribution strategies, key developments, and strategies, and future plans
- Key companies covered as a part of this study include Abbott Laboratories, Alcon Laboratories, Biomet Incorporated, Bausch and Lomb Incorporated, Boston Scientific Corporation, Johnson and Johnson, Medtronic Incorporated, St. Jude Medical Incorporated, Smith and Nephew PLC, Stryker Corporation, Synthes Incorporated, and Zimmer Holdings Incorporated.
- Insights from this report would allow marketers and the management authorities of companies to make informed decision regarding future product launches, technology up-gradation, market expansion, and marketing tactics
- The U.S. implantable medical devices market report caters to various stakeholders in this industry including investors, suppliers, product manufacturers, distributors, new entrants, and financial analysts.
- Stakeholders would have ease in decision-making through various strategy matrices used in analysing the U.S. Implantable Medical Devices Market.
Detailed Segmentation
- U.S. Implantable Medical Devices Market, By Implant Type:
- Orthopedic Implants
- Cardiovascular implants
- Dental Implants
- Intraocular Lens Implants
- Breast Implants
- Others
- U.S. Implantable Medical Devices Market, By End User:
- Hospitals
- Clinics
- Others
- Company Profiles
- Abbott Laboratories
- Alcon Laboratories
- Biomet Incorporated
- Bausch and Lomb Incorporated
- Boston Scientific Corporation
- Johnson and Johnson
- Medtronic Plc
- St. Jude Medical Incorporated
- Smith and Nephew PLC
- Stryker Corporation
- Synthes Incorporated
- Zimmer Holdings Incorporated
Table of Contents
1. Research Objective and Assumption
- Research Objectives
- Assumptions
- Abbreviations
2. Market Purview
- Report Description
- Market Definition and Scope
- Executive Summary
- Market Snippet, By Implant Type
- Market Snippet, By End User
- Coherent Opportunity Map (COM)
3. Market Dynamics, Regulations, and Trends Analysis
- Market Dynamics
- Drivers
- Restraints
- Market Opportunities
- Impact Analysis
- Key Developments
- Technological Advancement
- Regulatory Scenario
4. U.S. Implantable Medical Devices Market, By Implant Type, 2023-2030 (US$ Bn)
- Introduction
- Market Share Analysis, 2023 and 2030 (%)
- Y-o-Y Growth Analysis, 2018-2030
- Segment Trends
- Orthopaedic Implants
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018-2030, (US$ Bn)
- Segment Trends
- Cardiovascular implants
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018-2030, (US$ Bn)
- Segment Trends
- Dental Implants
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018-2030, (US$ Bn)
- Segment Trends
- Intraocular Lens Implants
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018-2030, (US$ Bn)
- Segment Trends
- Breast Implants
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018-2030, (US$ Bn)
- Segment Trends
- Others
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018-2030, (US$ Bn)
- Segment Trends
5. U.S. Implantable Medical Devices Market, By End User, 2023-2030 (US$ Bn)
- Introduction
- Market Share Analysis, 2023 and 2030 (%)
- Y-o-Y Growth Analysis, 2018-2030
- Segment Trends
- Hospitals
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018-2030, (US$ Bn)
- Clinics
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018-2030, (US$ Bn)
- Others
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018-2030, (US$ Bn)
6. Competitive Landscape
- Heat Map Analysis
- Company Profiles
- Abbott Laboratories
- Company Overview
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- SAlcon Laboratories
- Company Overview
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Biomet Incorporated
- Company Overview
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Bausch and Lomb Incorporated
- Company Overview
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Boston Scientific Corporation
- Company Overview
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Johnson and Johnson
- Company Overview
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Medtronic Plc
- Company Overview
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- St. Jude Medical Incorporated
- Company Overview
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Smith and Nephew PLC
- Company Overview
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Stryker Corporation
- Company Overview
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Synthes Incorporated
- Company Overview
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Zimmer Holdings Incorporated
- Company Overview
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
7. Section
- References
- Research Methodology
- About us and Sales Contact




