PUBLISHER: Coherent Market Insights | PRODUCT CODE: 1707354

PUBLISHER: Coherent Market Insights | PRODUCT CODE: 1707354
Global NPHP5 Retinal Degeneration Treatment Market, By Treatment Type, By Indication, By Distribution Channel, By Geography
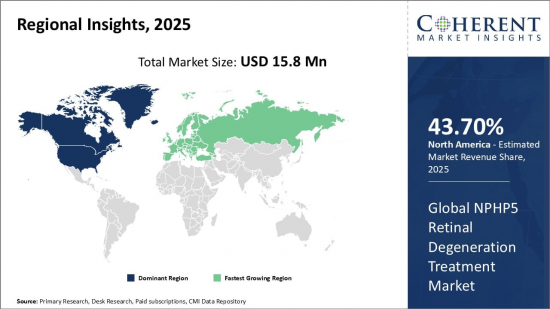
Global NPHP5 Retinal Degeneration Treatment Market is estimated to be valued at USD 15.8 Mn in 2025 and is expected to reach USD 133.3 Mn by 2032, growing at a compound annual growth rate (CAGR) of 35.6% from 2025 to 2032.
| Report Coverage | Report Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 15.8 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 35.60% | 2032 Value Projection: | USD 133.3 Mn |
NPHP5 retinal degeneration, also known as Senior-Loken syndrome 7, is a rare genetic ciliopathy that causes progressive visual impairment and can lead to complete blindness. It is caused by mutations in the NPHP5 gene, which encodes for nephrocystin-5, a protein critical for cilia function. The condition is characterized by retinitis pigmentosa, nephronophthisis, and other systemic involvement. Currently, there is no approved treatment for NPHP5 retinal degeneration, and management involves low vision aids and supportive care. However, recent research investigating the pathogenic mechanisms has uncovered potential drug targets such as cilia function, which is opening up new avenues for treatment development. Several biopharmaceutical companies have started clinical trials evaluating gene therapy and other novel therapeutic approaches to restore vision in these patients. Senior-Loken (S-L) is an autosomal recessive syndrome and a variant of the nephronophthisis-associated disorders, in which the cystic kidney disease is associated with retinal dystrophy (retinitis pigmentosa or Leber congenital amaurosis). It is a deleterious disease that culminates in blindness and renal failure.
Market Dynamics:
The NPHP5 retinal degeneration treatment market is driven by the high unmet medical need for an effective treatment for this debilitating condition. As per estimates, NPHP5 retinal degeneration has a prevalence of 1 in 200,000 live births globally. The increasing research focus on cilia pathways and recent breakthroughs in retinal gene therapy are further boosting market growth by expanding therapeutic opportunities. However, technical challenges associated with ocular gene delivery and the high costs of developing orphan disease therapies continue to restrain market revenues. On the other hand, partnerships between biotech firms and research institutions, growing public-private funding for rare disease, research and development present lucrative opportunities for market players.
Key features of the study:
- This report provides in-depth analysis of the global NPHP5 retinal degeneration treatment market, and provides market size (US$ Mn) and compound annual growth rate (CAGR %) for the forecast period (2025-2032), considering 2024 as the base year
- It elucidates potential revenue opportunities across different segments and explains attractive investment proposition matrices for this market
- This study also provides key insights about market drivers, restraints, opportunities, new product launches or approval, market trends, regional outlook, and competitive strategies adopted by key players
- It profiles key players in the global NPHP5 retinal degeneration treatment market based on the following parameters - company highlights, products portfolio, key highlights, financial performance, and strategies - Key companies covered as a part of this study include ProQR Therapeutics, Editas Medicine, Nanoscope Therapeutics, Inc., jCyte, Inc., Biogen, Novartis AG, Spark Therapeutics, MeiraGTx, NightstaRx, Beacon Therapeutics, Applied Genetic Technologies Corporation, ViGeneron and RetinAI Medical
- Insights from this report would allow marketers and the management authorities of the companies to make informed decisions regarding their future product launches, type up-gradation, market expansion, and marketing tactics
- The global NPHP5 retinal degeneration treatment market report caters to various stakeholders in this industry including investors, suppliers, product manufacturers, distributors, new entrants, and financial analysts
- Stakeholders would have ease in decision-making through various strategy matrices used in analyzing the global NPHP5 retinal degeneration treatment market
Detailed Segmentation:
- Global NPHP5 Retinal Degeneration Treatment Market, By Treatment Type
- Gene Therapy
- Cell Therapy
- Drug Therapy
- Others
- Global NPHP5 Retinal Degeneration Treatment Market, By Indication
- Retinitis Pigmentosa
- Leber Congenital Amaurosis
- Usher Syndrome
- Others
- Global NPHP5 Retinal Degeneration Treatment Market, By Distribution Channel
- Retail Pharmacies
- Hospital Pharmacies
- Online Pharmacies
- Global NPHP5 Retinal Degeneration Treatment Market, By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East
- Africa
- Company Profiles
- ProQR Therapeutics
- Editas Medicine
- Nanoscope Therapeutics, Inc.
- jCyte, Inc.
- Biogen
- Novartis AG
- Spark Therapeutics
- MeiraGTx
- NightstaRx
- Beacon Therapeutics
- Applied Genetic Technologies Corporation
- ViGeneron
- RetinAI Medical
Table of Contents
1. Research Objectives and Assumptions
- Research Objectives
- Assumptions
- Abbreviations
2. Market Purview
- Report Description
- Market Definition and Scope
- Executive Summary
- Market Snippet, By Treatment Type
- Market Snippet, By Indication
- Market Snippet, By Distribution Channel
- Market Snippet, By Region
- Coherent Opportunity Map (COM)
3. Market Dynamics, Regulations, and Trends Analysis
- Market Dynamics
- Increasing Launch of Awareness Campaigns
- Long approval timelines
- Emerging markets in Asia Pacific and Latin America
- Key Highlights
- Regulatory Scenario
- Market Trends
- PEST Analysis
- PORTER's Analysis
- Product Launches
- Epidemiology
- Mergers, Acquisitions, and Collaborations
4. Global NPHP5 Retinal Degeneration Treatment Market - Impact of Coronavirus (COVID-19) Pandemic
- COVID-19 Epidemiology
- Supply Side and Demand Side Analysis
- Economic Impact
5. Global NPHP5 Retinal Degeneration Treatment Market, By Treatment Type, 2020-2032, (US$ Mn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Gene Therapy
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Mn)
- Drug Therapy
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Mn)
- Others
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Mn)
6. Global NPHP5 Retinal Degeneration Treatment Market, By Indication, 2020-2032, (US$ Mn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Retinitis Pigmentosa
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Mn)
- Leber Congenital Amaurosis
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Mn)
- Usher Syndrome
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Mn)
- Others
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Mn)
7. Global NPHP5 Retinal Degeneration Treatment Market, By Distribution Channel, 2020-2032, (US$ Mn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Retail Pharmacies
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Mn)
- Hospital Pharmacies
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Mn)
- Online Pharmacies
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032,(US$ Mn)
8. Global NPHP5 Retinal Degeneration Treatment Market, By Region, 2020-2032, (US$ Mn)
- Introduction
- Market Share Analysis, By Region, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, For Region, 2021 -2032
- Country Trends
- North America
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Treatment Type, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Indication, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Distribution Channel, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020-2032,(US$ Mn)
- U.S.
- Canada
- Europe
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Treatment Type, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Indication, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Distribution Channel, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020-2032,(US$ Mn)
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Treatment Type, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Indication, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Distribution Channel, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020-2032,(US$ Mn)
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Latin America
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Treatment Type, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Indication, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Distribution Channel, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020-2032,(US$ Mn)
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Middle East
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Treatment Type, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Indication, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Distribution Channel, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020-2032,(US$ Mn)
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Treatment Type, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Indication, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Distribution Channel, 2020-2032,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Country/Region, 2020-2032,(US$ Mn)
- North Africa
- Central Africa
- South Africa
9. Competitive Landscape
- Company Profile
- ProQR Therapeutics
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Editas Medicine
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Nanoscope Therapeutics, Inc.
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- jCyte, Inc.
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Biogen
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Novartis AG
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Spark Therapeutics
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- MeiraGTx
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- NightstaRx
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Beacon Therapeutics
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Applied Genetic Technologies Corporation
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- ViGeneron
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- RetinAI Medical
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Analyst Views
10. Section
- References
- Research Methodology
- About us




