PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1782157

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1782157
Tuberculosis Diagnostics Test Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
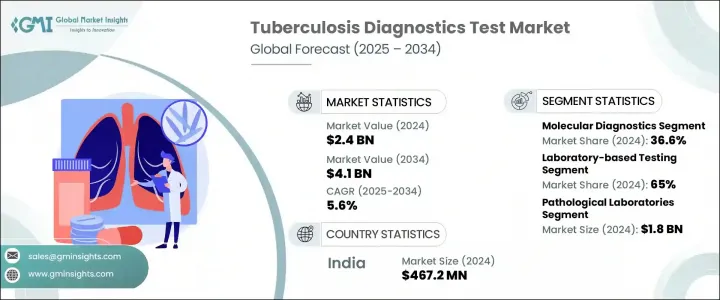
The Global Tuberculosis Diagnostics Test Market was valued at USD 2.4 billion in 2024 and is estimated to grow at a CAGR of 5.6% to reach USD 4.1 billion by 2034. Increasing TB prevalence worldwide, coupled with advancements in diagnostic technologies and a sharp rise in public health awareness, are fueling demand for reliable testing tools. The rising adoption of point-of-care testing and the implementation of structured screening programs are helping improve early detection rates. These efforts, supported by both public and private sectors, aim to streamline timely diagnosis and treatment, crucial steps in controlling the spread of tuberculosis.
A major driver behind the market's expansion is the global effort to strengthen early screening initiatives. Government-backed healthcare programs are rolling out structured strategies to support high-risk populations with better diagnostic access. These initiatives are accelerating early identification through community-based testing and outreach. Tuberculosis diagnostic tests are used to detect the presence of the mycobacterium tuberculosis bacteria and to determine if an individual has active TB or a latent TB infection, which is essential for guiding treatment decisions. With more emphasis on accurate identification, the market continues to witness greater investment in innovation and infrastructure across diagnostic platforms.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $2.4 Billion |
| Forecast Value | $4.1 Billion |
| CAGR | 5.6% |
In 2024, molecular diagnostics emerged as the leading segment, contributing 36.6% share and projected to grow at a CAGR of 5.9% through 2034. These diagnostics have changed the game by offering fast, sensitive, and accurate detection of Mycobacterium tuberculosis and its drug resistance traits. The use of polymerase chain reaction (PCR) as a central technique in this space enables healthcare providers to detect TB bacteria from clinical samples with precision, making early treatment decisions more effective.
The laboratory-based testing category held the largest share 65% in 2024. Its dominance is primarily attributed to the use of accurate, centralized testing methods critical for diagnosing complex and drug-resistant TB cases. These tests are typically performed in hospitals, public health institutions, and private certified laboratories. Common testing techniques include smear microscopy, culture-based diagnostics, and interferon-gamma release assays (IGRAs), all of which offer in-depth insight into disease severity and potential resistance, guiding clinicians in creating tailored treatment plans.
Asia Pacific Tuberculosis Diagnostics Test Market is expected to grow at a CAGR of 5.6% from 2025 to 2034. Factors contributing to this growth include the increasing number of TB cases, expanding public health education, greater access to diagnostic labs, and supportive governmental policies aimed at strengthening diagnostic infrastructure. As the region continues to invest in healthcare, the demand for advanced TB testing solutions is forecasted to rise.
Prominent players leading this space include Danaher Corporation, Abbott Laboratories, bioMerieux, Qiagen N.V., Becton, Dickinson and Company, and F. Hoffmann-La Roche. To strengthen their market presence, top firms are heavily investing in R&D to advance molecular and rapid diagnostic technologies. Strategic mergers and collaborations with healthcare providers and research institutions are helping companies expand their diagnostic portfolios and global footprint. Several players are focusing on creating low-cost, portable TB testing solutions tailored for low-resource settings, particularly in high-burden regions. Additionally, manufacturers are optimizing test sensitivity, reducing turnaround time, and ensuring their platforms meet international regulatory standards. These strategies collectively enhance accessibility, accuracy, and efficiency, positioning companies for long-term market leadership.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional
- 2.2.2 Test type
- 2.2.3 Modality
- 2.2.4 End use
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising burden of tuberculosis globally
- 3.2.1.2 Advancement in tuberculosis diagnostics techniques
- 3.2.1.3 Increasing awareness and screening programs regarding tuberculosis
- 3.2.1.4 Surge in point-of-care testing (POCT)
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Limited sensitivity and specificity
- 3.2.2.2 Stringent regulatory scenario
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Technological landscape
- 3.5 Regulatory landscape
- 3.5.1 North America
- 3.5.2 Europe
- 3.6 Future market trends
- 3.7 Pricing analysis
- 3.8 Porter’s analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Competitive analysis of major market players
- 4.4 Competitive positioning matrix
- 4.5 Strategy outlook matrix
Chapter 5 Market Estimates and Forecast, By Test Type, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Radiographic method
- 5.3 Diagnostic laboratory methods
- 5.3.1 Microscopy
- 5.3.2 Culture-based techniques
- 5.3.3 Serological tests
- 5.4 Molecular diagnostics
- 5.4.1 Polymerase chain reaction (PCR)
- 5.4.2 Nucleic acid amplification tests (NAAT)
- 5.4.3 GeneXpert MTB/RIF
- 5.5 Detection of latent infection
- 5.5.1 Tuberculin skin test (TST)/ Purified protein derivative (PPD)
- 5.5.1.1 First-generation PPD-based TSTs
- 5.5.1.2 New-generation skin tests with recombinant antigens
- 5.5.1 Tuberculin skin test (TST)/ Purified protein derivative (PPD)
- 5.6 Interferon-gamma release assays (IGRAs)
- 5.6.1.1 ELISA-based IGRAs
- 5.6.1.2 ELISPOT-based IGRAs
- 5.6.1.3 Point-of-care IGRAs
- 5.7 Cytokine detection assays
- 5.8 Detection of drug resistance (DST)
- 5.9 Phage assay
- 5.10 Other test types
Chapter 6 Market Estimates and Forecast, By Modality, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Point of care testing (POCT)
- 6.3 Laboratory-based testing (Non-POCT)
Chapter 7 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Pathological laboratories
- 7.3 Other end use
Chapter 8 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 Japan
- 8.4.3 India
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 Abbott Laboratories
- 9.2 Anhui Zhifei Longcom Biopharmaceutical Co.
- 9.3 Becton, Dickinson and Company
- 9.4 bioMerieux
- 9.5 Danaher Corporation (Cepheid)
- 9.6 F. Hoffmann-La Roche
- 9.7 Generium Pharmaceuticals
- 9.8 Hain Lifescience
- 9.9 Hologic
- 9.10 Japan BCG Laboratory
- 9.11 NIPRO
- 9.12 Oxford Immunotec
- 9.13 Qiagen N.V.
- 9.14 Sanofi
- 9.15 Siemens Healthineers
- 9.16 Thermo Fisher Scientific




