PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1766338

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1766338
Shock Wave Therapy Devices Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
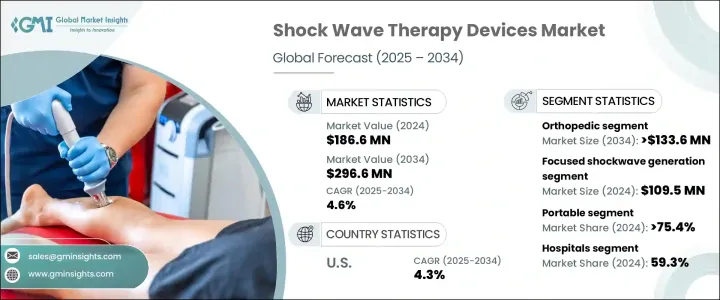
The Global Shock Wave Therapy Devices Market was valued at USD 186.6 million in 2024 and is estimated to grow at a CAGR of 4.6% to reach USD 296.6 million by 2034. This growth is primarily driven by the increasing prevalence of musculoskeletal disorders, a growing preference for non-invasive treatments, and a rising number of sports-related injuries. Conditions such as tendinitis, plantar fasciitis, and calcific tendonitis have become more common worldwide due to sedentary lifestyles, sports injuries, and an aging population. As more individuals experience these musculoskeletal issues, the demand for effective therapeutic techniques like shock wave therapy is on the rise.
Shock wave therapy devices are medical instruments designed to deliver acoustic waves to specific areas of the body for therapeutic purposes. These devices utilize various technologies, including electrohydraulic, electromagnetic, and piezoelectric mechanisms, to generate shock waves. The application of shock wave therapy has expanded beyond traditional orthopedic and musculoskeletal treatments to include fields such as urology, cardiology, wound care, erectile dysfunction treatments, and even aesthetic medicine. Recent advancements have led to the development of radial pressure wave devices and electromagnetic systems tailored for specific clinical applications.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $186.6 Million |
| Forecast Value | $296.6 Million |
| CAGR | 4.6% |
Additionally, the introduction of handheld and portable shock wave devices has made it possible to administer treatments in outpatient and home care settings. These devices often feature digital interfaces, pre-programmed treatment protocols, and real-time feedback systems, enhancing ease of use for clinicians and improving consistency and reliability during therapy sessions. The integration of artificial intelligence-based diagnostic support and robotic-assisted mechanisms has further enhanced targeting accuracy and treatment planning, driving broader clinical adoption by reducing procedural variability and improving success rates.
The focused shock wave generation segment accounted for USD 109.5 million in 2024. Focused shock wave therapy delivers high-intensity shock waves to targeted tissues, minimizing damage to surrounding healthy tissues. This precision enhances therapeutic effects and treatment outcomes, leading to increased patient satisfaction. Moreover, focused shock wave therapy reduces the risk of tissue damage, bruising, and nerve irritation, improving the safety and tolerability of the treatment. These factors are expected to influence the segment's growth in the market.
The orthopedic application segment is anticipated to grow at a CAGR of 4.9% and reach USD 133.6 million by 2034. Shock wave therapy offers a non-invasive alternative to traditional surgical treatments for orthopedic disorders, reducing associated risks, recovery times, and complications. Conditions such as chronic tendinopathies and musculoskeletal pain syndromes are prevalent in orthopedics and often resist conventional treatments. Shock wave therapy provides an effective solution for these chronic and treatment-resistant orthopedic ailments, promoting tissue repair, breaking down calcifications, and alleviating pain, thereby improving patient outcomes and overall quality of life.
U.S. Shock Wave Therapy Devices Market generated USD 68 million in 2024 and is projected to grow at a CAGR of 4.3% between 2025 and 2034. The United States leads globally in technological innovation and healthcare, owing to substantial investments in medical equipment research, development, and commercialization. Manufacturers in the region continuously innovate and enhance shock wave therapy devices through the application of new technologies, significantly boosting market growth by precisely improving treatment outcomes. Furthermore, the increasing body of clinical evidence supporting the efficacy of shock wave therapy across various medical disciplines, along with endorsements from professional societies and healthcare organizations, drives the growing adoption of shock wave therapy devices in clinical practice.
Prominent players operating in the Global Shock Wave Therapy Devices Industry include BTL Industries, Boston Scientific Corporation, Dornier MedTech, EMS Electro Medical Systems S.A., Lumenis Ltd., Olympus Corporation, Siemens Healthineers AG, and Zimmer MedizinSysteme GmbH. Companies in the shock wave therapy devices market adopt various strategies to strengthen their presence and market position. These strategies include continuous innovation in product development, focusing on enhancing device efficiency, portability, and user-friendliness. Manufacturers often collaborate with healthcare providers, physiotherapy clinics, and sports centers to expand their reach and promote the application of shock wave therapy.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional
- 2.2.2 Technology
- 2.2.3 Application
- 2.2.4 Type
- 2.2.5 End use
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing prevalence of musculoskeletal disorders
- 3.2.1.2 Technological advancements in shock wave therapy devices
- 3.2.1.3 Rising number of sports injuries
- 3.2.1.4 Growing preference for non-invasive treatments
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of shock wave therapy devices
- 3.2.3 Market opportunities
- 3.2.3.1 Rising demand in aesthetic and dermatology applications
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.4.4 Latin America
- 3.4.5 Middle East & Africa
- 3.5 Technology and innovation landscape
- 3.5.1 Current technological trends
- 3.5.2 Emerging technologies
- 3.6 Pricing analysis
- 3.7 Gap analysis
- 3.8 Porter's analysis
- 3.9 Pipeline analysis
- 3.10 PESTEL analysis
- 3.11 Value chain analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Company market share analysis
- 4.3.1 By region
- 4.3.1.1 North America
- 4.3.1.2 Europe
- 4.3.1.3 Asia Pacific
- 4.3.1 By region
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Mergers & acquisitions
- 4.6.2 Partnerships & collaborations
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Technology, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Focused shockwave generation
- 5.2.1 Electrohydraulic shock wave generation (EHSG)
- 5.2.2 Piezoelectric shock wave generation (PSG)
- 5.2.3 Electromagnetic shock wave generation (EMSG)
- 5.3 Radial or ballistic shock wave generation
- 5.4 Combined shock wave generation
Chapter 6 Market Estimates and Forecast, By Application, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Orthopedic
- 6.3 Cardiology
- 6.4 Urology
- 6.5 Other applications
Chapter 7 Market Estimates and Forecast, By Type, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Portable
- 7.3 Fixed
Chapter 8 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Hospitals and clinics
- 8.3 Diagnostic laboratories
- 8.4 Ambulatory surgical centers
Chapter 9 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 Japan
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 BD
- 10.2 Boston Scientific
- 10.3 BTL
- 10.4 Dornier MedTech
- 10.5 EMS
- 10.6 Focal One
- 10.7 Johnson & Johnson
- 10.8 LUMENIS
- 10.9 OLYMPUS
- 10.10 SIEMENS Healthineers
- 10.11 Zimmer MedizinSysteme




