PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1699286

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1699286
Contract Research Organization (CRO) Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025-2034
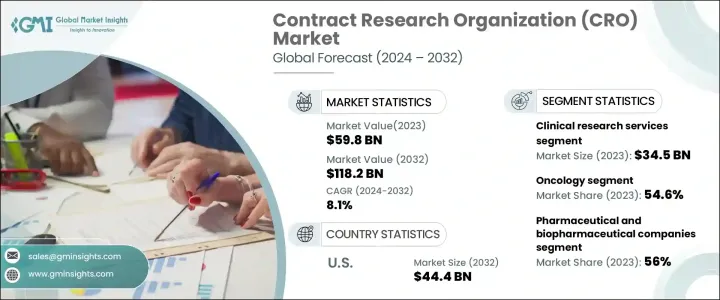
The Global Contract Research Organization Market reached USD 59.8 billion in 2023 and is on track to expand at a CAGR of 8.1% from 2024 to 2032. The demand for outsourced research services is surging as pharmaceutical and biotechnology firms seek cost-effective solutions to accelerate drug development and meet stringent regulatory requirements. With a growing number of clinical trials worldwide, CROs are playing a crucial role in streamlining drug discovery, reducing operational costs, and enhancing research efficiency. Investments in medical research continue to rise, further strengthening the market's growth trajectory.
The increasing prevalence of chronic diseases is fueling the need for innovative treatments, driving pharmaceutical companies to rely on specialized research firms. CROs offer expertise in clinical trials, regulatory compliance, and laboratory services, making them indispensable to the pharmaceutical and biotech sectors. Outsourcing research allows companies to focus on innovation while leveraging the infrastructure and experience of CROs to navigate complex regulatory landscapes. Additionally, advancements in precision medicine, gene therapy, and biologics are expanding the scope of contract research services, creating new opportunities for industry players. The global shift toward personalized healthcare is further amplifying the demand for specialized research, with CROs facilitating the development of targeted therapies and cutting-edge medical solutions.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $59.8 Billion |
| Forecast Value | $118.2 Billion |
| CAGR | 8.1% |
The market is segmented by service type into early phase development, clinical research, laboratory, and regulatory consulting services. Clinical research services led the industry, generating USD 34.5 billion in revenue in 2023. This segment continues to thrive as the demand for innovative treatments and diagnostics rises. Clinical research is structured into four phases, each essential in bringing new therapies to market. The increase in chronic disease cases is intensifying the need for clinical trials, prompting pharmaceutical firms to expand their research efforts. Outsourcing research enables companies to optimize their resources, accelerate drug approval processes, and enhance efficiency in clinical trials.
The contract research organization market is also categorized by therapeutic area, including oncology, cardiology, infectious diseases, neurology, gastroenterology, hepatology, ophthalmology, and others. Oncology dominated the market in 2023 with a commanding 54.6% share and is expected to grow at a CAGR of 8.5% through 2032. The rising incidence of cancer has intensified research efforts to develop advanced therapies and medical devices, leading to an upsurge in clinical trial activity. The push for breakthrough treatments is driving investment in oncology research, leading to a significant increase in demand for contract research services. As new therapies emerge, oncology-focused trials are expanding rapidly, solidifying the role of CROs in the drug development ecosystem.
North America contract research organization market was valued at USD 22.6 billion in 2023, with projections indicating growth to USD 44.4 billion by 2032. The presence of major pharmaceutical and biotechnology firms in the region has contributed to its strong market share. As drug development becomes increasingly complex, the need for specialized expertise and state-of-the-art infrastructure continues to grow. CROs are instrumental in advancing medical research by providing comprehensive research solutions, regulatory support, and data-driven insights, positioning them as key players in the evolving pharmaceutical landscape.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing R&D expenditure worldwide
- 3.2.1.2 Rising number of clinical trials in emerging economies
- 3.2.1.3 Growing outsourcing of R&D activities
- 3.2.1.4 Rising technological advancements
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Intellectual property right issues
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Clinical trial analysis
- 3.4.1 Clinical trial volume, by region, 2018 - 2023
- 3.4.2 Clinical trial volume, by phase of development, 2018 - 2023
- 3.4.3 Clinical trial volume, by indication, 2018 - 2023
- 3.5 Regulatory landscape
- 3.5.1 U.S.
- 3.5.2 Europe
- 3.5.3 Asia Pacific
- 3.5.3.1 Singapore
- 3.5.3.2 Malaysia
- 3.5.3.3 Indonesia
- 3.5.3.4 Thailand
- 3.5.3.5 South Korea
- 3.5.3.6 Philippines
- 3.6 Clinical trials- Asia Pacific advantage
- 3.7 Drug discovery and development process
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2023
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Competitive market share analysis
- 4.4 Strategy dashboard
- 4.5 Merger and acquisition landscape
- 4.5.1 Merger and acquisition deals in the CRO industry, 2018 – 2023
- 4.5.2 Completed contract research organization merger and acquisition deals, 2018 - 2023
- 4.5.3 Influence of private equity in CRO mergers and acquisitions
- 4.5.4 Impact of industry consolidation on the overall market
- 4.5.5 Analyst insights
Chapter 5 Market Estimates and Forecast, By Service Type, 2021 – 2032 ($ Mn)
- 5.1 Key trends
- 5.2 Early phase development services
- 5.2.1 Discovery phase
- 5.2.2 Chemistry, manufacturing and control (CMC)
- 5.2.3 Preclinical services
- 5.2.3.1 Pharmacokinetic/pharmacodynamic (PK/PD)
- 5.2.3.2 Toxicology testing services
- 5.2.3.3 Other preclinical services
- 5.3 Clinical research services
- 5.3.1 Phase I
- 5.3.2 Phase II
- 5.3.3 Phase III
- 5.3.4 Phase IV
- 5.4 Laboratory services
- 5.4.1 Bioanalytical testing services
- 5.4.2 Analytical testing services
- 5.4.2.1 Physical characterization
- 5.4.2.2 Raw material testing
- 5.4.2.3 Batch release testing
- 5.4.2.4 Stability testing
- 5.4.2.5 Other analytical testing services
- 5.5 Regulatory consulting services
Chapter 6 Market Estimates and Forecast, By Therapeutic Area, 2021 – 2032 ($ Mn)
- 6.1 Key trends
- 6.2 Oncology
- 6.3 Clinical pharmacology
- 6.4 Cardiology
- 6.5 Infectious disease
- 6.6 Neurology
- 6.7 Gastroenterology and hepatology
- 6.8 Ophthalmology
- 6.9 Other therapeutic areas
Chapter 7 Market Estimates and Forecast, By End Use, 2021 – 2032 ($ Mn)
- 7.1 Key trends
- 7.2 Pharmaceutical and biopharmaceutical companies
- 7.3 Medical device companies
- 7.4 Academic institutes
Chapter 8 Market Estimates and Forecast, By Region, 2021 – 2032 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 Japan
- 8.4.3 India
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.6 Middle East and Africa
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 Caidya
- 9.2 Charles River Laboratories
- 9.3 CMIC HOLDINGS
- 9.4 EPS Holdings
- 9.5 ICON plc
- 9.6 IQVIA (Quintiles IMS)
- 9.7 Iris Pharma
- 9.8 Laboratory Corporation of America Holdings
- 9.9 Lexitas Pharma Services
- 9.10 Medpace Holdings
- 9.11 Ora
- 9.12 Parexel International (MA)
- 9.13 Pharmaceutical Product Development (Thermo Fisher Scientific)
- 9.14 Premier Research
- 9.15 ProTrials Research
- 9.16 Syneos Health
- 9.17 TFS HealthScience
- 9.18 The Emmes Company
- 9.19 Trial Runners
- 9.20 Veristat
- 9.21 Worldwide Clinical Trials
- 9.22 WuXi Clinical (WuXi AppTec)




