PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1716609

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1716609
Cardiac Rhythm Management Devices Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
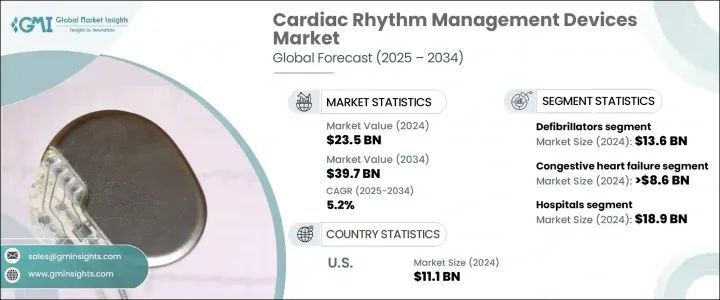
The Global Cardiac Rhythm Management Devices Market reached USD 23.5 billion in 2024 and is projected to expand at a CAGR of 5.2% between 2025 and 2034. The growth of this market is fueled by the rising incidence of cardiovascular diseases worldwide, including heart failure, arrhythmias, and other rhythm-related disorders. The steady increase in aging populations across major economies is also driving market expansion, as older individuals are more prone to cardiac complications. Factors such as sedentary lifestyles, poor dietary habits, and rising cases of hypertension, diabetes, and obesity are further intensifying the need for advanced cardiac care solutions.
With cardiovascular diseases remaining the leading cause of mortality worldwide, there is a growing demand for innovative and effective cardiac rhythm management devices that can improve patient outcomes and enhance quality of life. Additionally, constant advancements in medical technology, including remote monitoring and next-generation implantable devices, are transforming the way healthcare providers manage and treat cardiac rhythm disorders. The surge in healthcare expenditure, coupled with greater awareness about early diagnosis and preventive cardiac care, is expected to open up new opportunities for market players. The integration of artificial intelligence (AI) and machine learning (ML) into cardiac rhythm management systems is also anticipated to drive future market trends by enabling predictive analytics and personalized treatment options.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $23.5 Billion |
| Forecast Value | $39.7 Billion |
| CAGR | 5.2% |
The market comprises a wide range of product segments, including pacemakers, defibrillators, and cardiac resynchronization therapy (CRT) devices. Among these, defibrillators hold the largest market share, contributing significantly to the overall revenue. Defibrillators, especially implantable cardioverter defibrillators (ICDs), are vital for patients at high risk of life-threatening arrhythmias. These devices are designed to monitor heart rhythms continuously and deliver electric shocks when abnormal rhythms are detected, thereby preventing sudden cardiac death. The demand for defibrillators is expected to rise steadily as cardiovascular conditions continue to be a leading cause of morbidity and mortality globally.
In terms of application, the cardiac rhythm management devices market is segmented into congestive heart failure, arrhythmias, bradycardia, and tachycardia. Among these, congestive heart failure accounted for USD 8.6 billion in 2024. Devices like ICDs and CRT systems play a crucial role in managing heart failure by improving heart rhythm coordination, enhancing cardiac output, and alleviating symptoms such as fatigue and breathlessness, thereby improving patient quality of life.
The United States Cardiac Rhythm Management Devices Market generated USD 11.1 billion in 2024, dominating the global landscape. The country's advanced healthcare infrastructure, coupled with favorable reimbursement frameworks and broad access to state-of-the-art treatment options, continues to propel market growth. Strong clinical research, ongoing product innovations, and significant investments in R&D are enabling the rapid adoption of CRM devices in the U.S., solidifying its position as a global leader in this space.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Growing prevalence of heart failure and other cardiac disorders
- 3.2.1.2 Technological advancements and introduction of innovative devices for cardiac rhythm monitoring
- 3.2.1.3 Increasing public awareness
- 3.2.1.4 Rising sedentary lifestyle
- 3.2.1.5 Favorable reimbursement scenario
- 3.2.1.6 Growing geriatric population base coupled with rising prevalence of obesity
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of devices
- 3.2.2.2 Product recalls
- 3.2.2.3 Stringent regulatory approvals
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Reimbursement scenario
- 3.6 Technology landscape
- 3.7 Future market trends
- 3.8 Pricing analysis, 2024
- 3.9 Pipeline products
- 3.10 Indication landscape
- 3.11 Number of units, 2021 - 2034
- 3.11.1 Pacemaker
- 3.11.2 Defibrillators
- 3.11.3 CRT devices
- 3.12 Porter's analysis
- 3.13 GAP analysis
- 3.14 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Competitive analysis of major market players
- 4.4 Company market share analysis
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Product, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Pacemakers
- 5.2.1 Implantable pacemakers
- 5.2.2 External pacemakers
- 5.3 Defibrillators
- 5.3.1 Implantable cardioverter defibrillator (ICDs)
- 5.3.1.1 Transvenous implantable cardioverter defibrillator
- 5.3.1.2 Subcutaneous implantable cardioverter defibrillator
- 5.3.1.2.1 Single-chamber ICDs
- 5.3.1.2.2 Dual-chamber ICDs
- 5.3.2 External defibrillator
- 5.3.2.1 Manual external defibrillator
- 5.3.2.2 Automated external defibrillator
- 5.3.2.2.1 Semi-automated external defibrillator
- 5.3.2.2.2 Fully automated external defibrillator
- 5.3.2.3 Wearable cardioverter defibrillator
- 5.3.1 Implantable cardioverter defibrillator (ICDs)
- 5.4 Cardiac resynchronization therapy devices
- 5.4.1 Cardiac resynchronization therapy devices- D
- 5.4.2 Cardiac resynchronization therapy devices- P
Chapter 6 Market Estimates and Forecast, By Application, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Congestive heart failure
- 6.3 Arrhythmias
- 6.4 Bradycardia
- 6.5 Tachycardia
- 6.6 Other applications
Chapter 7 Market Estimates and Forecast, By End Use, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Hospitals
- 7.3 Cardiac care centers
- 7.4 Ambulatory surgical centers
- 7.5 Other end use
Chapter 8 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 Japan
- 8.4.3 India
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 Abbott
- 9.2 ABIOMED
- 9.3 Amiitalia
- 9.4 Asahi Kasei
- 9.5 BIOTRONIK
- 9.6 Boston Scientific
- 9.7 BPL Medical Technologies
- 9.8 CU Medical
- 9.9 Defibtech
- 9.10 LivaNova
- 9.11 Medico
- 9.12 Medtronic
- 9.13 MicroPort
- 9.14 Nihon Kohden
- 9.15 Osypka Medical
- 9.16 Pacetronix
- 9.17 Philips
- 9.18 Schiller
- 9.19 Stryker
- 9.20 Vitatron




