PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1721443

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1721443
Radiology Report Quality Assurance Services Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
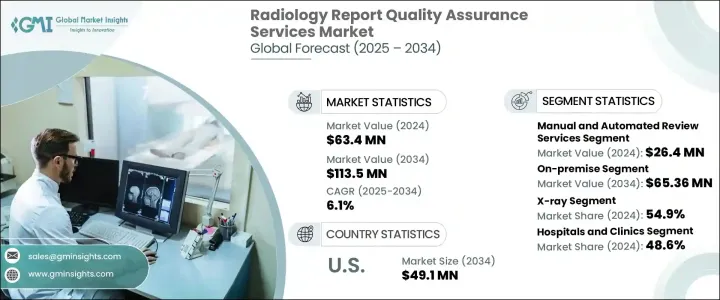
The Global Radiology Report Quality Assurance Services Market was valued at USD 63.4 million in 2024 and is estimated to grow at a CAGR of 6.1% to reach USD 113.5 million by 2034. With the increasing dependence on diagnostic imaging across various clinical disciplines, the importance of accuracy in radiology reporting has become a central concern. Healthcare providers, teleradiology networks, and diagnostic imaging centers are placing greater emphasis on error reduction and patient safety, driving demand for robust radiology report quality assurance (QA) services. These services play a pivotal role in validating the accuracy, completeness, and clinical consistency of radiology interpretations, ensuring that diagnostic outcomes align with high clinical standards. As imaging becomes an integral part of patient management and treatment pathways, the market is gaining momentum due to the rising need to reduce misdiagnoses, improve clinical decisions, and support better healthcare outcomes.
Growing pressure to eliminate discrepancies in diagnostic reports, especially in high-volume imaging environments, continues to fuel the market. Radiology QA services are increasingly being adopted as hospitals and diagnostic centers transition to value-based care models. These frameworks rely on consistent, high-quality interpretations to minimize risks and improve reimbursement outcomes. The market is witnessing a strategic shift, with providers not only focusing on error detection but also on enhancing workflow efficiency and audit transparency. With technological advancements and rising regulatory scrutiny, stakeholders are adopting QA programs to ensure compliance with national and international clinical guidelines.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $63.4 Million |
| Forecast Value | $113.5 Million |
| CAGR | 6.1% |
Errors such as misinterpretations, annotation inaccuracies, and miscommunications in radiology findings are intensifying the need for quality assurance mechanisms. Both manual and automated review services are being integrated into diagnostic workflows. The manual review services segment generated USD 26.4 million in 2024, indicating strong demand for expert human insight in complex or borderline imaging cases. While AI-powered tools have shown potential in routine error detection, human-led audits remain indispensable, especially in nuanced clinical scenarios or when a second opinion is required.
The market is also segmented by deployment model, with the on-premise solutions segment accounting for a 58.5% share in 2024. Many healthcare institutions prefer on-premise systems due to their ability to offer greater control over infrastructure, enhanced data security, and customization according to internal policies. Facilities with in-house IT departments and stringent compliance mandates are particularly inclined toward these systems to meet their operational and regulatory goals.
The United States Radiology Report Quality Assurance Services Market is projected to reach USD 49.1 million by 2034. Increasing pressure to eliminate diagnostic errors and a growing focus by regulatory agencies on standardized reporting are among the primary growth drivers. Hospitals and clinics across the U.S. are embracing QA solutions to meet compliance standards and elevate clinical quality.
Leading companies in the global market include RadNet, ONRAD, Aster Medical Imaging, PROBICS Informatics Solutions, National Diagnostic Imaging, HealthLevel, INFINITT North America, Teleradiology Solutions, Intelerad, Ventura Teleradiology, Envision Healthcare, Virtual Radiologic (vRad), Vesta Teleradiology, PurDes Radiology, and RamSoft. These companies are strengthening their market positions by enhancing AI-integrated QA platforms, supporting remote teleradiology operations, and tailoring quality protocols to fit diverse imaging modalities. Strategic alliances with imaging providers, investments in cloud-based deployment, and increased interoperability with PACS/RIS systems are helping streamline diagnostic workflows and improve report accuracy.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Growing incidence of radiology reporting errors
- 3.2.1.2 Rising demand to comply with regulatory requirements
- 3.2.1.3 Emphasis on value-based care
- 3.2.1.4 Growing demand to efficiently manage increasing burden on radiology labs
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of peer-review services
- 3.2.2.2 Limited availability of skilled radiologists.
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technological landscape
- 3.6 Future market trends
- 3.7 Gap analysis
- 3.8 Porter’s analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Service Type, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Manual and automated review services
- 5.3 Audit and compliance services
- 5.4 Error detection and correction services
- 5.5 Other service types
Chapter 6 Market Estimates and Forecast, By Deployment Type, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 On-premise
- 6.3 Cloud-based
Chapter 7 Market Estimates and Forecast, By Modality, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 CT scan
- 7.3 X-ray
- 7.4 MRI
- 7.5 Ultrasound
- 7.6 Mammography
- 7.7 PET-CT
Chapter 8 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Hospitals and clinics
- 8.3 Medical imaging centers
- 8.4 Teleradiology corporations
- 8.5 Other end use
Chapter 9 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 India
- 9.4.3 Japan
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 Saudi Arabia
- 9.6.2 South Africa
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Aster Medical Imaging
- 10.2 Envision Healthcare
- 10.3 HealthLevel
- 10.4 INFINITT North America
- 10.5 Intelerad
- 10.6 National Diagnostic Imaging
- 10.7 ONRAD
- 10.8 PROBICS Informatics Solutions
- 10.9 PurDes Radiology
- 10.10 RadNet
- 10.11 RamSoft
- 10.12 Teleradiology Solutions
- 10.13 Ventura Teleradiology Services
- 10.14 Vesta Teleradiology
- 10.15 Virtual Radiologic (vRad)




