PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1721607

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1721607
In-vitro Diagnostics Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
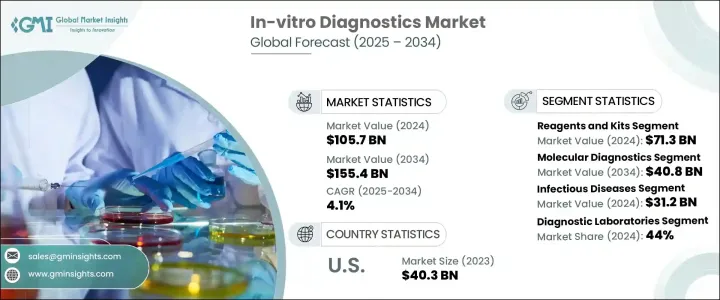
The Global In-Vitro Diagnostics Market was valued at USD 105.7 billion in 2024 and is estimated to grow at a CAGR of 4.1% to reach USD 155.4 billion by 2034. In-vitro diagnostics (IVD) includes a wide range of medical devices, reagents, and systems used to analyze biological samples such as blood, urine, and tissues outside the human body. These tools play a vital role in detecting, diagnosing, and managing diseases, enabling faster and more accurate clinical decisions. As healthcare systems around the world continue to shift toward preventive care and precision medicine, the demand for advanced diagnostic tools is rising steadily.
IVD solutions are increasingly being adopted not only in hospitals and clinical laboratories but also in home care and point-of-care settings. The growing burden of chronic and infectious diseases, rising awareness about early disease detection, and the emergence of novel diagnostic technologies are collectively driving the expansion of this market. Moreover, the adoption of artificial intelligence and digital health platforms in diagnostics is transforming the way results are generated, interpreted, and delivered, creating new avenues for personalized and real-time care. Supportive regulatory policies, favorable reimbursement scenarios, and a strong push for healthcare digitization across developed and emerging economies further accelerate the industry's growth trajectory.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $105.7 Billion |
| Forecast Value | $155.4 Billion |
| CAGR | 4.1% |
The reagents and kits segment generated USD 71.3 billion in 2024 and is expected to grow at a CAGR of 4.4% from 2025 to 2034. This growth is largely driven by the increasing need for precise and early-stage diagnosis, as well as the rising demand for customized treatment plans. Reagents and kits cover a broad spectrum of products, including biochemical reagents, antibodies, assay kits, probes, and primers, all of which are essential for running a variety of diagnostic tests. With the advancement of molecular diagnostics, these products are now capable of delivering highly accurate and faster results compared to traditional testing methods, making them indispensable in modern diagnostic labs.
The molecular diagnostics segment held a 25.5% market share in 2024 and is expected to grow significantly, reaching USD 40.8 billion by 2034. This segment benefits from ongoing innovations that enhance test accuracy, turnaround time, and ease of use. Molecular diagnostic tools are now the preferred option for detecting infectious diseases, genetic disorders, and cancer, thanks to their ability to identify pathogens and genetic mutations at a molecular level. Unlike conventional tests that often require days to return results, molecular diagnostics can produce actionable insights within hours or even minutes, enabling faster treatment decisions and better patient outcomes.
The North America In-Vitro Diagnostics Market generated USD 45.1 billion in 2024, maintaining a dominant position due to strong healthcare infrastructure, a high burden of chronic illnesses, and early adoption of advanced technologies. Despite a stringent regulatory landscape, the region continues to support the approval and commercialization of cutting-edge diagnostic solutions.
Key players in the Global In-Vitro Diagnostics Market include Abbott Laboratories, ACON Laboratories, Agilent Technologies, Becton, Dickinson and Company, Bio-Rad Laboratories, Dragerwerk, F. Hoffmann-La Roche, Danaher Corporation, Medtronic, Meridian Bioscience, Nova Biomedical, PerkinElmer, Siemens Healthineers, and Sysmex Corporation. These companies focus on research and development, strategic partnerships, and global expansion to meet the rising demand for advanced diagnostic technologies.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising prevalence of infectious and chronic diseases worldwide
- 3.2.1.2 Growing adoption and rising demand for personalized medicine
- 3.2.1.3 Increasing adoption of point-of-care testing in developing countries
- 3.2.1.4 Surging number of pathology labs and services equipped with advanced diagnostics machine
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of diagnostic services
- 3.2.2.2 Unfavorable reimbursement scenario
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technological landscape
- 3.6 Future market trends
- 3.7 Gap analysis
- 3.8 Porter’s analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Product, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Reagents and kits
- 5.3 Instruments
Chapter 6 Market Estimates and Forecast, By Test Type, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Clinical chemistry
- 6.3 Immunoassay/immunochemistry
- 6.4 Molecular diagnostics
- 6.5 Hematology
- 6.6 Urinalysis
- 6.7 Other test types
Chapter 7 Market Estimates and Forecast, By Application, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Oncology
- 7.3 Infectious diseases
- 7.4 Diabetes
- 7.5 Cardiology
- 7.6 Nephrology
- 7.7 Autoimmune diseases
- 7.8 Drug testing/pharmacogenomics
- 7.9 Other applications
Chapter 8 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Hospitals
- 8.3 Diagnostic laboratories
- 8.4 Academic and research institutes
- 8.5 Other end use
Chapter 9 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 India
- 9.4.3 Japan
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 Saudi Arabia
- 9.6.2 South Africa
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Abbott Laboratories
- 10.2 ACON Laboratories
- 10.3 Agilent Technologies
- 10.4 Becton, Dickinson and Company
- 10.5 Biomerieux
- 10.6 Bio-Rad Laboratories
- 10.7 Dragerwerk
- 10.8 F. Hoffmann-La Roche
- 10.9 Danaher Corporation
- 10.10 Medtronic
- 10.11 Meridian Bioscience
- 10.12 Nova Biomedical
- 10.13 PerkinElmer
- 10.14 Siemens Healthineers
- 10.15 Sysmex Corporation




