PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1750272

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1750272
Continuous Bladder Irrigation Devices Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
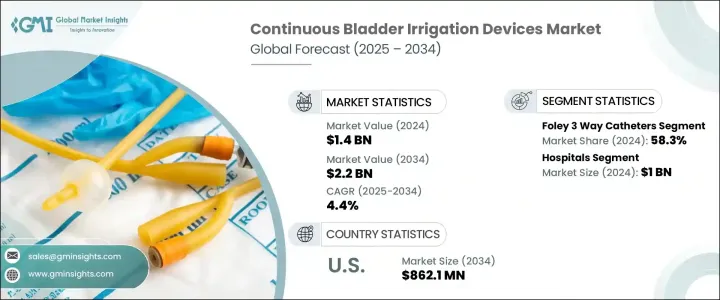
The Global Continuous Bladder Irrigation Devices Market was valued at USD 1.4 billion in 2024 and is estimated to grow at a CAGR of 4.4% to reach USD 2.2 billion by 2034, driven by the increasing prevalence of urological conditions such as bladder cancer, hematuria, and benign prostatic hyperplasia (BPH). As healthcare providers aim to reduce complications and improve patient recovery, CBI systems have become essential in hospitals and clinical settings. These systems are particularly vital during post-operative care following urologic surgeries, as they help prevent clot formation and maintain catheter function.
Technological progress in catheter materials, design, and irrigation delivery systems has significantly transformed the functionality and reliability of continuous bladder irrigation (CBI) devices. Innovations such as anti-kink tubing, pressure-controlled flow mechanisms, and improved balloon designs have enhanced patient safety and clinician ease-of-use. These advancements contribute to better control of urine flow, reduced catheter blockages, and more effective clot management. Additionally, modern CBI systems incorporate features that minimize the risk of contamination, supporting hospitals' efforts to reduce catheter-associated urinary tract infections (CAUTIs). Healthcare protocols now place a strong emphasis on standardized irrigation practices, as a result, advanced CBI devices have become a critical component in maintaining patient outcomes in post-operative and long-term urological care.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $1.4 Billion |
| Forecast Value | $2.2 Billion |
| CAGR | 4.4% |
Foley 3-way catheters held the largest market share at 58.3% in 2024, reflecting their widespread adoption in managing post-surgical bleeding and maintaining catheter patency. These catheters are capable of irrigation, balloon inflation, and urine drainage, making them indispensable in treating complex urological conditions. Beyond their post-operative use, these catheters are also utilized in trauma-related bladder injuries, chemotherapy-induced cystitis, and other urinary retention complications, which have widened their application in healthcare worldwide.
The Hospitals segment in the continuous bladder irrigation devices market held the largest share in 2024 due to the high frequency of procedures like bladder tumor resections and prostate surgeries, which require consistent irrigation to prevent blood clot retention. With an increasing focus on reducing catheter-associated urinary tract infections (CAUTIs), hospitals are investing more in advanced CBI systems that support patient safety and surgical success. The role of continuous bladder irrigation in minimizing post-surgical complications has made it a standard element in inpatient urological care.
United States Continuous Bladder Irrigation Devices Market is expected to reach USD 569.3 million in 2024, driven by the country's expanding elderly population is experiencing higher rates of prostate enlargement, bladder cancer, and hematuria-conditions that often require long-term irrigation solutions. This demographic trend has directly contributed to an increase in bladder-related surgeries, such as transurethral procedures, which require reliable post-surgical irrigation. Hospitals and surgical centers prioritize high-quality irrigation systems that offer durability, infection resistance, and ease of maintenance.
Key players such as Cardinal Health, C. R. Bard, Sterimed, Baxter, Advin Health Care, Teleflex, Angiplast, HEMC, Medline, Bactiguard, Boston Scientific, Coloplast, AdvaCare, B. Braun, and Vogt Medical are adopting focused strategies to enhance market presence. These include product innovation in catheter materials, strategic partnerships with healthcare institutions, and expansion into emerging markets. Companies are also emphasizing infection-control features, offering customizable solutions, and investing in R&D to meet regulatory standards and improve care outcomes.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising prevalence of urological disorders
- 3.2.1.2 Advancements in catheter materials and irrigation technologies
- 3.2.1.3 Growing adoption of minimally invasive urological procedures
- 3.2.1.4 Growing geriatric population
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Risk of catheter-associated urinary tract infections
- 3.2.2.2 Lack of awareness and limited access to advanced urological care in LMICs
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Trump administration tariffs
- 3.4.1 Impact on trade
- 3.4.1.1 Trade volume disruptions
- 3.4.1.2 Retaliatory measures
- 3.4.2 Impact on the Industry
- 3.4.2.1 Supply-side impact (raw materials)
- 3.4.2.1.1 Price volatility in key materials
- 3.4.2.1.2 Supply chain restructuring
- 3.4.2.1.3 Production cost implications
- 3.4.2.2 Demand-side impact (selling price)
- 3.4.2.2.1 Price transmission to end markets
- 3.4.2.2.2 Market share dynamics
- 3.4.2.2.3 Consumer response patterns
- 3.4.2.1 Supply-side impact (raw materials)
- 3.4.3 Key companies impacted
- 3.4.4 Strategic industry responses
- 3.4.4.1 Supply chain reconfiguration
- 3.4.4.2 Pricing and product strategies
- 3.4.4.3 Policy engagement
- 3.4.5 Outlook and future considerations trump administration tariffs
- 3.4.1 Impact on trade
- 3.5 Future market trends
- 3.6 Regulatory landscape
- 3.7 Porter's analysis
- 3.8 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Competitive dashboard
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategic outlook matrix
Chapter 5 Market Estimates and Forecast, By Product, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Foley 3 way catheters
- 5.2.1 Silicone catheters
- 5.2.2 Latex catheters
- 5.3 Accessories
- 5.3.1 Irrigation set
- 5.3.2 Roller clamp
- 5.3.3 Outlet bags
- 5.4 Irrigation bags
Chapter 6 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Hospitals
- 6.3 Long term care facilities
- 6.4 Other end use
Chapter 7 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 North America
- 7.2.1 U.S.
- 7.2.2 Canada
- 7.3 Europe
- 7.3.1 Germany
- 7.3.2 UK
- 7.3.3 France
- 7.3.4 Italy
- 7.3.5 Spain
- 7.3.6 Netherlands
- 7.4 Asia Pacific
- 7.4.1 Japan
- 7.4.2 China
- 7.4.3 India
- 7.4.4 Australia
- 7.4.5 South Korea
- 7.5 Latin America
- 7.5.1 Mexico
- 7.5.2 Brazil
- 7.5.3 Argentina
- 7.6 Middle East and Africa
- 7.6.1 South Africa
- 7.6.2 Saudi Arabia
- 7.6.3 UAE
Chapter 8 Company Profiles
- 8.1 AdvaCare
- 8.2 Advin Health Care
- 8.3 Angiplast
- 8.4 B. Braun
- 8.5 Bactiguard
- 8.6 Baxter
- 8.7 Boston Scientific
- 8.8 C. R. Bard
- 8.9 Cardinal Health
- 8.10 Coloplast
- 8.11 HEMC
- 8.12 Medline
- 8.13 Sterimed
- 8.14 Teleflex
- 8.15 Vogt Medical




