PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1750466

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1750466
Ablation Catheters Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
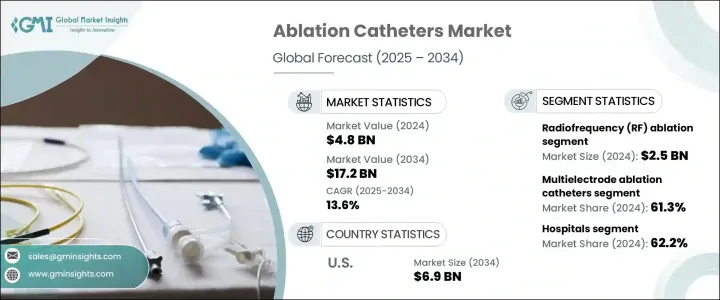
The Global Ablation Catheters Market was valued at USD 4.8 billion in 2024 and is estimated to grow at a CAGR of 13.6% to reach USD 17.2 billion by 2034, driven by the increasing prevalence of atrial fibrillation (AFib) and other arrhythmias worldwide. Technological advancements in catheter design, including improvements in tip design, contact force technology, and new energy modalities like cryoablation and pulsed field ablation, are enhancing the precision and safety of these procedures. These innovations have not only expanded the clinical applications of ablation catheters but also boosted physician confidence, resulting in greater adoption across various healthcare settings.
Ablation catheters are vital instruments in modern cardiac care, specifically designed to treat arrhythmias, which are irregular heartbeats. These catheters work by delivering targeted energy to specific areas of the heart tissue that are causing abnormal electrical signals. Depending on the type of energy used, these catheters can apply radiofrequency (RF) energy, cryothermal energy, or pulsed electric fields to create controlled lesions in the heart tissue, effectively disrupting the faulty electrical pathways. This targeted destruction of tissue helps restore a normal heart rhythm, offering patients an alternative to long-term medication or more invasive surgical procedures. Radiofrequency ablation is the most used method, where heat is applied to the heart tissue, creating lesions that block or modify abnormal electrical signals.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $4.8 Billion |
| Forecast Value | $17.2 Billion |
| CAGR | 13.6% |
The market is divided into multielectrode and single-point ablation catheters. The multielectrode segment dominated in 2024, holding a 61.3% share. This segment is seeing increased demand, particularly due to the rising incidence of AFib and the need for catheters that can map and treat simultaneously. Multielectrode catheters offer several advantages, including faster procedural times, reduced anesthesia requirements, and improved clinical outcomes, making them an attractive option in high-volume electrophysiology labs.
In terms of technology, radiofrequency (RF) ablation remains the dominant method, contributing USD 2.5 billion in 2024. RF ablation is widely regarded as the gold standard in cardiac rhythm management due to its reliability, safety, and ability to produce permanent lesions in the heart. RF ablation catheters also benefit from advancements such as temperature-controlled energy delivery, irrigated tips, and contact force sensing, which improve both safety and efficacy during procedures.
United States Ablation Catheters Market generated USD 2 billion in 2024, driven by the increasing incidence of cardiovascular diseases, including AFib, largely due to an aging population and lifestyle-related risk factors such as hypertension and diabetes. This growing patient pool has led to higher demand for catheter-based ablation procedures, further fueled by favorable insurance reimbursement policies and healthcare system support for arrhythmia treatments.
The leading companies in the ablation catheters market include Abbott Laboratories, AngioDynamics, AtriCure, Biotronik, Boston Scientific, and Medtronic. These companies continue to innovate, pushing the boundaries of technology to develop safer and more effective devices. Key strategies adopted by major companies in the ablation catheters market include continuous technological innovation, strategic partnerships, and expanding their product portfolios. Companies like Boston Scientific and Medtronic are focused on improving catheter efficiency by integrating advanced features such as contact force sensing and temperature-controlled energy delivery. These innovations aim to enhance procedural outcomes and patient safety.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising prevalence of atrial fibrillation and other cardiac arrhythmias
- 3.2.1.2 Technological advancements in ablation catheter design
- 3.2.1.3 Growing preference for minimally invasive procedures
- 3.2.1.4 Favorable reimbursement policies
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of ablation catheter procedures and equipment
- 3.2.2.2 Risk of complications such as cardiac perforation or thromboembolism
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Trump administration tariffs
- 3.4.1 Impact on trade
- 3.4.1.1 Trade volume disruptions
- 3.4.1.2 Retaliatory measures
- 3.4.2 Impact on the Industry
- 3.4.2.1 Supply-side impact (raw materials)
- 3.4.2.1.1 Price volatility in key materials
- 3.4.2.1.2 Supply chain restructuring
- 3.4.2.1.3 Production cost implications
- 3.4.2.2 Demand-side impact (selling price)
- 3.4.2.2.1 Price transmission to end markets
- 3.4.2.2.2 Market share dynamics
- 3.4.2.2.3 Consumer response patterns
- 3.4.2.1 Supply-side impact (raw materials)
- 3.4.3 Key companies impacted
- 3.4.4 Strategic industry responses
- 3.4.4.1 Supply chain reconfiguration
- 3.4.4.2 Pricing and product strategies
- 3.4.4.3 Policy engagement
- 3.4.5 Outlook and future considerations
- 3.4.1 Impact on trade
- 3.5 Technology landscape
- 3.6 Future market trends
- 3.7 Regulatory landscape
- 3.8 Patent analysis
- 3.9 Porter's analysis
- 3.10 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Competitive market share analysis
- 4.3 Competitive dashboard
- 4.4 Company matrix analysis
- 4.5 Competitive analysis of major market players
- 4.6 Competitive positioning matrix
- 4.7 Strategic outlook matrix
Chapter 5 Market Estimates and Forecast, By Product Type, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Multielectrode ablation catheters
- 5.3 Single point ablation catheters
Chapter 6 Market Estimates and Forecast, By Technology, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Radiofrequency (RF) ablation
- 6.3 Cryoablation
- 6.4 Pulse field ablation
Chapter 7 Market Estimates and Forecast, By End Use, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Hospitals
- 7.3 Ambulatory surgical centers
- 7.4 Other end use
Chapter 8 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Italy
- 8.3.5 Spain
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 Japan
- 8.4.2 China
- 8.4.3 India
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Mexico
- 8.5.2 Brazil
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 Abbott Laboratories
- 9.2 AngioDynamics
- 9.3 AtriCure
- 9.4 Biotronik
- 9.5 Boston Scientific
- 9.6 CardioFocus
- 9.7 CathRx
- 9.8 Imricor Medical Systems
- 9.9 Japan Lifeline
- 9.10 Johnson & Johnson
- 9.11 Lepu Medical
- 9.12 Medtronic
- 9.13 MicroPort
- 9.14 Osypka
- 9.15 Stryker




