PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1797835

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1797835
Single-use Duodenoscope Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
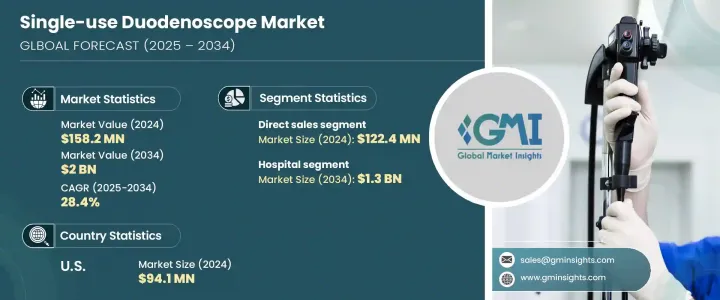
The Global Single-Use Duodenoscope Market was valued at USD 158.2 million in 2024 and is estimated to grow at a CAGR of 28.4% to reach USD 2 billion by 2034. The growth is driven by the rising demand for infection control, technological improvements in device design, and the increasing prevalence of gastrointestinal conditions. Healthcare providers are turning to disposable duodenoscopes to improve patient safety and reduce the risk of cross-contamination, a concern that continues to gain traction across clinical settings.
As more hospitals and clinics adopt advanced endoscopic solutions, these devices are being used more frequently, especially in light of their one-time use format, which eliminates the need for complex sterilization procedures and reduces infection risks. Improvements in imaging compatibility, better material choices, and patient-centric designs are also accelerating the adoption of these disposable tools. With the healthcare sector increasingly focused on reducing hospital-acquired infections, the transition to single-use solutions is becoming a top priority.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $158.2 Million |
| Forecast Value | $2 Billion |
| CAGR | 28.4% |
The market's growth is further supported by continuous product innovation to improve diagnostic accuracy and clinical outcomes. Single-use duodenoscopes are specifically engineered for endoscopic procedures in the duodenum, offering a safer alternative to reusable models. Once used, these devices are discarded, eliminating any reprocessing-related issues. Recent enhancements in design have enabled easier integration with existing imaging platforms, delivering sharper, real-time visuals during procedures and resulting in more precise diagnoses. Healthcare professionals prefer disposable models as they reduce procedural complexity and eliminate contamination risks associated with reprocessing reusable scopes. Greater reliability and ease of use make these devices an integral part of endoscopic procedures.
The video duodenoscope segment generated substantial revenue in 2024. These scopes have gained wide acceptance because they deliver high-definition visuals when paired with in-house imaging systems. Their real-time imaging capabilities allow clinicians to spot even minor anomalies in the gastrointestinal tract with enhanced clarity. In addition to improving diagnostic accuracy, these video scopes reduce the likelihood of infection by eliminating the cleaning and disinfection process, which has historically posed contamination risks. As healthcare systems adopt advanced video technologies, including HD and UHD formats, the demand for disposable video duodenoscopes is projected to rise further. Their ability to provide a sterile, reliable diagnostic tool makes them essential for safe endoscopic procedures.
The direct sales segment contributed USD 122.4 million in 2024 and is anticipated to grow at a CAGR of 29% through 2034. The direct sales channel enables manufacturers to build closer relationships with healthcare professionals, offering tailored support, real-time feedback, and customized training. This collaborative approach ensures a better understanding of end-user requirements and accelerates the delivery of technical support and product improvements. For medical devices like duodenoscopes, direct engagement ensures precision in customer service and strengthens brand loyalty. It also allows companies to receive immediate clinical feedback, adapt product strategies, and optimize future product designs based on market needs.
United States Single-Use Duodenoscope Market reached USD 94.1 million in 2024 and is projected to grow at a CAGR of 29.2% between 2025 and 2034. The demand is rising due to favorable reimbursement trends and a growing emphasis on minimizing infection risks. Healthcare providers in the region are shifting away from reusable options toward disposable alternatives that support infection control efforts. The increasing burden of gastrointestinal diseases, including bile duct blockages, pancreatitis, and GI cancers, is creating a strong demand for safe and effective diagnostic tools. Coupled with policy support and advancements in device technology, the U.S. continues to contribute to overall market expansion.
Prominent companies leading the Global Single-Use Duodenoscope Market include PENTAX MEDICAL, FUJIFILM, Boston Scientific, Ambu, STORZ, OLYMPUS, and AOHUA. These companies are actively pursuing strategic moves to strengthen their position in the growing market. They focus on continuous product upgrades, integrating high-definition video technologies, and launching models with improved ergonomics and ease of use. Many are expanding their direct sales force to enhance customer engagement and post-sale service. In addition, collaborative R&D efforts and strategic partnerships with healthcare facilities help these players stay ahead of technological demands and clinical needs. Streamlining manufacturing processes, securing regulatory approvals, and scaling production capabilities form their strategy to capture greater market share.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional
- 2.2.2 Sales channel
- 2.2.3 End use
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.1.1 Supplier landscape
- 3.1.2 Value addition at each stage
- 3.1.3 Factor affecting the value chain
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing prevalence of gastrointestinal disorders
- 3.2.1.2 Technological advancements
- 3.2.1.3 Rising awareness among patients and healthcare professionals
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost associated with device
- 3.2.3 Market opportunities
- 3.2.3.1 Integration with AI and smart imaging systems
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.5 Porter's analysis
- 3.6 PESTEL analysis
- 3.7 Technology and innovation landscape
- 3.7.1 Current technological trends
- 3.7.2 Emerging technologies
- 3.8 Single-use duodenoscope, by region, volume analysis (2021 -2034)
- 3.8.1 Global
- 3.8.2 North America
- 3.8.3 Europe
- 3.9 Price trends
- 3.9.1 By region
- 3.9.2 By product
- 3.10 Future market trends
- 3.11 Gap analysis
- 3.12 Brand analysis
- 3.13 Reimbursement scenario
- 3.13.1 Impact of reimbursement policies on market growth
- 3.14 Patent Landscape
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.2.1 Global
- 4.2.2 North America
- 4.2.3 Europe
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Mergers & acquisitions
- 4.6.2 Partnerships & collaborations
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Sales Channel, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Direct sales
- 5.3 In-direct sales
Chapter 6 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Hospitals
- 6.3 Ambulatory surgical centers
- 6.4 Other end use
Chapter 7 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 North America
- 7.2.1 U.S.
- 7.2.2 Canada
- 7.3 Europe
- 7.3.1 Germany
- 7.3.2 UK
- 7.3.3 France
- 7.3.4 Spain
- 7.3.5 Italy
- 7.3.6 Netherlands
- 7.3.7 Rest of Europe
- 7.4 Rest of the World
Chapter 8 Company Profiles
- 8.1 Ambu
- 8.2 Boston Scientific




