PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1755288

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1755288
VMAT2 Inhibitors Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
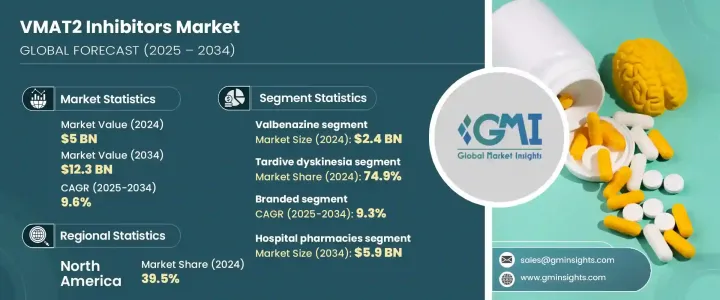
The Global VMAT2 Inhibitors Market was valued at USD 5 billion in 2024 and is estimated to grow at a CAGR of 9.6% to reach USD 12.3 billion by 2034, driven by the growing incidence of movement-related neurological disorders, particularly Tardive Dyskinesia (TD) and Huntington's Disease (HD). These conditions require targeted therapies to manage motor symptoms, increasing reliance on this drug class. Additionally, introducing better-tolerated and more effective VMAT2 inhibitors, combined with broader regulatory approvals and ongoing R&D, continues to push the market forward. Pharma companies are leveraging commercial strategies, policy support, and expanding patient outreach to improve drug accessibility and deepen market reach.
VMAT2 inhibitors work by decreasing the levels of neurotransmitters like dopamine, serotonin, and norepinephrine, helping to manage hyperkinetic movement disorders. These drugs block the transport of monoamines into synaptic vesicles, helping to regulate excess dopamine activity that contributes to involuntary motor issues. Used primarily in conditions such as Huntington's Disease (HD) and Tardive Dyskinesia (TD), these therapies offer a promising solution for managing debilitating symptoms that significantly impair patients' quality of life. By regulating excessive dopamine activity in the brain, VMAT2 inhibitors help control involuntary movements, muscle spasms, and other motor dysfunctions commonly seen in these disorders.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $5 Billion |
| Forecast Value | $12.3 Billion |
| CAGR | 9.6% |
Valbenazine segment led the market with a valuation of USD 2.4 billion in 2024 supported by its strong efficacy profile, favorable safety record, and simple dosing regimen, all of which have contributed to high prescription volumes. As one of the first VMAT2 inhibitors approved for TD, it secured early market presence, brand credibility, and has since expanded its use across additional indications. Its user-friendly treatment schedule further enhances patient compliance and drives sustained demand.
The Tardive Dyskinesia segment accounted for 74.9% share in 2024 fueled by the increasing diagnosis of TD in patients treated long-term with antipsychotics for psychiatric conditions. The approval and marketing of VMAT2 inhibitors specifically designed to address TD has significantly improved treatment outcomes. Rising awareness and screening efforts are further enabling timely intervention, contributing to the increased uptake of these therapies.
U.S. VMAT2 Inhibitors Market reached USD 1.8 billion in 2024 driven by widespread access to psychiatric care, advanced diagnostics, and strong reimbursement structures. A mature healthcare infrastructure and patient-friendly insurance models continue to facilitate the adoption of VMAT2 inhibitors across various care settings. The high rate of diagnosis and treatment for neuropsychiatric disorders such as schizophrenia, bipolar disorder, and major depressive disorder contributes significantly to market demand, as these conditions are commonly associated with drug-induced movement disorders like Tardive Dyskinesia.
Key players in the Global VMAT2 Inhibitors Market include: Neurocrine Biosciences, Teva Pharmaceutical Industries, Lupin, Dr. Reddy's Laboratories, H. Lundbeck, Intas Pharmaceuticals Ltd, Bausch Health Companies, APOTEX, Mylan, Actavis Labs, BionPharma, and Upsher-Smith Laboratories. Leading companies in the VMAT2 Inhibitors Market are focusing on expanding therapeutic applications, enhancing product formulations, and scaling up manufacturing capabilities. Many are investing in clinical trials to gain regulatory approval for additional indications beyond TD and HD. Strategic collaborations with healthcare providers and insurers are also helping to improve drug accessibility and patient adherence. Firms like Intas, Bausch Health Companies, and Neurocrine Biosciences are bolstering their portfolios with next-generation therapies and differentiated dosing strategies.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing prevalence of Tardive Dyskinesia (TD) and Huntington’s disease
- 3.2.1.2 Advancements in novel drug formulations
- 3.2.1.3 Increased awareness and diagnosis of movement disorders
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of drugs
- 3.2.2.2 Side effects associated with VMAT2 inhibitors
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Trump administration tariffs
- 3.5.1 Impact on trade
- 3.5.1.1 Trade volume disruptions
- 3.5.1.2 Retaliatory measures
- 3.5.2 Impact on the industry
- 3.5.2.1 Supply-side impact (raw materials)
- 3.5.2.1.1 Price volatility in key materials
- 3.5.2.1.2 Supply chain restructuring
- 3.5.2.1.3 Production cost implications
- 3.5.2.2 Demand-side impact (selling price)
- 3.5.2.2.1 Price transmission to end markets
- 3.5.2.2.2 Market share dynamics
- 3.5.2.2.3 Consumer response patterns
- 3.5.2.1 Supply-side impact (raw materials)
- 3.5.3 Key companies impacted
- 3.5.4 Strategic industry responses
- 3.5.4.1 Supply chain reconfiguration
- 3.5.4.2 Pricing and product strategies
- 3.5.4.3 Policy engagement
- 3.5.5 Outlook and future considerations
- 3.5.1 Impact on trade
- 3.6 Future market trends
- 3.7 Pipeline analysis
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Competitive analysis of major market players
- 4.4 Competitive positioning matrix
- 4.5 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Drug, 2021-2034 ($ Mn)
- 5.1 Key trends
- 5.2 Valbenazine
- 5.3 Deutetrabenazine
- 5.4 Tetrabenazine
Chapter 6 Market Estimates and Forecast, By Application, 2021-2034 ($ Mn)
- 6.1 Key trends
- 6.2 Tardive dyskinesia (TD)
- 6.3 Huntington's disease
Chapter 7 Market Estimates and Forecast, By Type, 2021-2034 ($ Mn)
- 7.1 Key trends
- 7.2 Branded
- 7.3 Generic
Chapter 8 Market Estimates and Forecast, By Distribution Channel, 2021-2034 ($ Mn)
- 8.1 Key trends
- 8.2 Hospital pharmacies
- 8.3 Retail pharmacies
- 8.4 Online pharmacies
Chapter 9 Market Estimates and Forecast, By Region, 2021-2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 Japan
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Actavis Labs
- 10.2 APOTEX
- 10.3 Bausch Health Companies
- 10.4 BionPharma
- 10.5 Dr. Reddy's Laboratories
- 10.6 H. Lundbeck
- 10.7 Intas Pharmaceuticals Ltd
- 10.8 Lupin
- 10.9 Mylan
- 10.10 Neurocrine Biosciences
- 10.11 Teva Pharmaceutical Industries
- 10.12 Upsher-Smith Laboratories




