PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1755387

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1755387
Veterinary Active Pharmaceutical Ingredients (API) Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
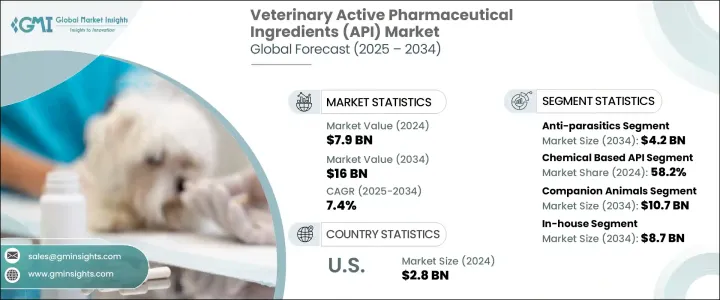
The Global Veterinary Active Pharmaceutical Ingredients (API) Market was valued at USD 7.9 billion in 2024 and is estimated to grow at a CAGR of 7.4% to reach USD 16 billion by 2034. This steady growth is largely fueled by increasing pet ownership, rising awareness about animal health, and advancements in veterinary technologies. Consumers are becoming more inclined to treat animals with the same level of healthcare they expect for themselves, which has directly impacted the demand for quality veterinary medications. The growing prevalence of zoonotic diseases has also intensified the urgency for reliable treatments, making veterinary APIs more crucial than ever in the development of safe and effective drugs.
Veterinary APIs are core ingredients used to formulate medicines that treat, manage, or prevent diseases in animals. These ingredients are essential for both livestock and companion animals, serving a wide range of therapeutic applications. With the increase in global livestock production and pet adoption, there is a consistent need for high-quality APIs that meet regulatory benchmarks such as Good Manufacturing Practices (GMP). APIs are cost-effective due to their compatibility with bulk production, ensuring price efficiency without compromising quality. They also allow pharmaceutical manufacturers to tailor drugs for specific species and dosage forms, including oral, injectable, and feed-based solutions. The flexibility, affordability, and high consistency of APIs make them integral to producing effective veterinary treatments.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $7.9 Billion |
| Forecast Value | $16 Billion |
| CAGR | 7.4% |
The market is segmented by API type into anti-parasitics, anti-infectives, vaccines, biologics, hormones, anti-inflammatories, and other APIs. Among these, anti-parasitics dominated the market with a revenue of USD 2 billion in 2024 and are forecasted to reach USD 4.2 billion by 2034, recording a CAGR of 7.9%. The increasing incidence of parasitic diseases in animals, such as heartworms, fleas, and gastrointestinal infections, is driving the demand for targeted anti-parasitic treatments, which in turn pushes the need for robust and quality APIs in this segment.
Based on synthesis type, the market is categorized into chemical-based APIs, biological APIs, and highly potent APIs (HPAPIs). Chemical-based APIs held the largest share of 58.2% in 2024 due to their cost-efficiency, long shelf life, and ease of mass production. These APIs are widely used in treating a range of conditions like infections and inflammation and serve as the foundational building blocks for many veterinary drugs. Their pharmacological properties make them suitable for combination with other ingredients, enhancing their therapeutic efficacy. Continuous improvements in chemical synthesis technologies are also supporting the increased adoption of these APIs across various veterinary applications.
In terms of animal type, the market is segmented into livestock animals and companion animals. The companion animals segment is projected to grow at a CAGR of 7.6%, reaching USD 10.7 billion by 2034. This growth is attributed to the increasing trend of pet humanization and the willingness of pet owners to invest in preventive and therapeutic healthcare. Demand for advanced veterinary pharmaceuticals such as biologics, hormones, and anti-inflammatory drugs continues to rise as pet care becomes more specialized and sophisticated.
The market is further divided by service type into in-house manufacturing and contract outsourcing. In-house manufacturing held the largest market share at 55.3% in 2024 and is projected to reach USD 8.7 billion by 2034. This segment benefits from advantages like enhanced quality assurance, lower production costs, and faster time-to-market for drug development. Companies with in-house API production capabilities are better positioned to manage inventory and adjust to market demands quickly, giving them a competitive edge in the fast-evolving veterinary pharmaceutical space.
Regionally, North America accounted for the highest revenue of USD 3.1 billion in 2024 and is expected to climb to USD 6 billion by 2034, growing at a CAGR of 7.1%. The region's leadership is reinforced by widespread pet ownership, high awareness of animal health issues, and a strong veterinary healthcare infrastructure. The demand for premium-quality APIs continues to increase as more consumers seek advanced treatments for both pets and livestock, supporting North America's dominant position in the global market.
Key players actively contributing to the market landscape include AMGIS Lifescience, Abino Pharma, BOC Sciences, Hikal, Huvepharma (Olon), Boehringer Ingelheim, Indukern Group, NGL Fine-Chem, Menadiona, Ofichem Group, Qilu Pharmaceutical, Sequent Scientific, Procyon Life Sciences, SUANFARMA, Vetpharma, and Veyx-Pharma. These companies are focused on strengthening their manufacturing capabilities, expanding their product portfolios, and meeting rising global demand for veterinary APIs.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising pet ownership rate
- 3.2.1.2 Growing initiative and awareness for animal health
- 3.2.1.3 Growing technological advancements in drug formulations
- 3.2.1.4 Rising incidence of zoonotic disease
- 3.2.1.5 Expanding outsourcing services
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Stringent regulatory approval
- 3.2.2.2 High R&D cost
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Porter's analysis
- 3.6 PESTLE analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Competitive analysis of major market players
- 4.4 Competitive positioning matrix
- 4.5 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By API Type, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Anti-infectives
- 5.3 Anti-parasitics
- 5.4 Vaccines
- 5.5 Hormones
- 5.6 Biologics
- 5.7 Anti-inflammatory
- 5.8 Other API types
Chapter 6 Market Estimates and Forecast, By Synthesis Type, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Chemical based API
- 6.3 Biological API
- 6.4 Highly potent API (HPAPI)
Chapter 7 Market Estimates and Forecast, By Animal Type, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Companion animals
- 7.3 Livestock animals
Chapter 8 Market Estimates and Forecast, By Service Type, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 In-house
- 8.3 Contract outsourcing
- 8.3.1 Contract development
- 8.3.2 Contract manufacturing
Chapter 9 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 India
- 9.4.3 Japan
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Abino Pharma
- 10.2 AMGIS Lifescience
- 10.3 BOC Sciences.
- 10.4 Boehringer Ingelheim
- 10.5 Hikal
- 10.6 Huvepharma
- 10.7 Indukern Group
- 10.8 Menadiona
- 10.9 NGL Fine-Chem
- 10.10 Ofichem Group
- 10.11 Procyon Life Sciences
- 10.12 Qilu Pharmaceutical
- 10.13 Sequent Scientific
- 10.14 SUANFARMA
- 10.15 Vetpharma
- 10.16 Veyx-Pharma




