PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1766241

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1766241
Docetaxel Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
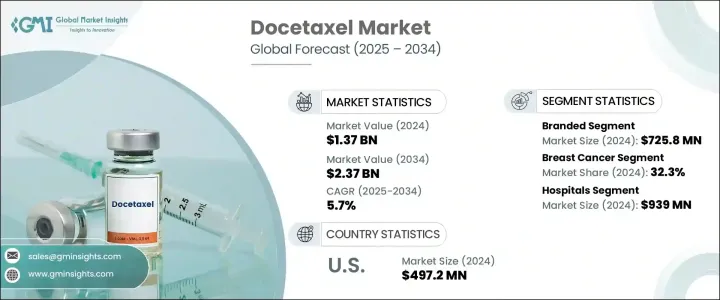
The Global Docetaxel Market was valued at USD 1.37 billion in 2024 and is estimated to grow at a CAGR of 5.7% to reach USD 2.37 billion by 2034. This growth is largely driven by the increasing incidence of various cancers, including breast, lung, prostate, and gastric cancers, where docetaxel is widely used. As a second-generation taxane, docetaxel stabilizes microtubules and halts the division of cancer cells, making it a crucial part of chemotherapy regimens. It is particularly effective in combination therapies, helping to improve survival rates for patients with advanced cancers, such as triple-negative breast cancer and castration-resistant prostate cancer. Additionally, innovations in drug delivery methods, such as nanoparticle formulations and liposomal encapsulations, have improved their bioavailability and reduced toxicity, expanding their clinical applications.
The growing approvals for biosimilars and generic docetaxel are making the drug more accessible, particularly in lower-middle-income regions, while the increasing focus on oncology R&D boosts demand for this essential cytotoxic agent. Digital health technologies also play a role by enabling better treatment personalization through remote monitoring of treatment responses and side effects. Furthermore, greater cancer awareness, urbanization, and early diagnosis efforts are contributing to the broader reliance on chemotherapy and drugs like docetaxel.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $1.37 Billion |
| Forecast Value | $2.37 Billion |
| CAGR | 5.7% |
The branded segment of the docetaxel market generated USD 725.8 million in 2024, benefiting from the established clinical reputation and efficacy of original formulations. Despite the presence of generics, branded products continue to dominate in developed markets due to their trusted safety profiles, regulatory approvals, and high-quality standards. Branded docetaxel is often preferred by oncologists for treating complex cancers such as breast cancer, non-small cell lung cancer (NSCLC), and prostate cancer, particularly in hospital settings. Moreover, the investment in new delivery methods and combination therapies further strengthens the brand's value.
In 2024, breast cancer segment held 32.3% driven by early and advanced treatment regimens for HER2-negative and triple-negative breast cancer patients. It is commonly used in combination treatments like TAC (docetaxel, doxorubicin, and cyclophosphamide), which have been shown to improve survival outcomes. Advances in personalized oncology systems continue to increase the demand for docetaxel in managing breast cancer cases.
U.S. Docetaxel Market was valued at USD 497.2 million in 2024. Factors such as favorable reimbursement policies, growing precision oncology services, and a focus on personalized medicine are fueling the demand. Furthermore, the expansion of outpatient chemotherapy access, increasing cancer awareness, and continuous clinical research are reinforcing the U.S. market's growth. The role of the U.S. as a key market is further strengthened by strategic partnerships between pharmaceutical companies and research centers, which accelerate drug development and market growth.
Leading players in the Global Docetaxel Industry include Alchem International, Alkem Labs, Arch Pharmalabs, Aspen Pharmacare, Cipla, Cisen Pharmaceutical, LGM Pharma, Phyton Biotech, Qilu Pharmaceutical, Teva Active Pharmaceutical Ingredients (TAPI), Teva Pharmaceuticals, Venus Remedies, and Xiromed. Companies operating in the docetaxel market are focusing on expanding their product portfolios by offering new formulations, delivery methods, and combination therapies. Partnerships with research institutions and hospitals are vital to enhancing the clinical applications and adoption of docetaxel. Many players are investing in biosimilars and generics to tap into cost-sensitive markets, ensuring broader access to this essential drug. Additionally, the development of personalized medicine and advanced digital health technologies allows companies to better cater to patient needs, improving treatment outcomes.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional
- 2.2.2 Product type
- 2.2.3 Indication
- 2.2.4 End use
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.1.1 Supplier landscape
- 3.1.2 Value addition at each stage
- 3.1.3 Factor affecting the value chain
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising global cancer prevalence
- 3.2.1.2 Increasing adoption of combination therapies
- 3.2.1.3 Favorable regulatory approvals and guidelines
- 3.2.1.4 Technological advancements in drug formulation
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Severe side effects and toxicity concerns
- 3.2.2.2 Patent expirations and generic competition
- 3.2.3 Market opportunities
- 3.2.3.1 Expansion of personalized oncology treatment approaches
- 3.2.3.2 Increasing investments in oncology research and development
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.5 Porter's analysis
- 3.6 PESTEL analysis
- 3.7 Technology and innovation landscape
- 3.8 Price trends
- 3.8.1 By region
- 3.8.2 By product type
- 3.9 Future market trends
- 3.10 Reimbursement scenario
- 3.10.1 Impact of reimbursement policies on market growth
- 3.11 Consumer behaviour analysis
- 3.12 Trade statistics (HS code)
- 3.12.1 Major importing countries
- 3.12.2 Major exporting countries
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Mergers and acquisitions
- 4.6.2 Partnerships and collaborations
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Product Type, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Branded
- 5.3 Generics
Chapter 6 Market Estimates and Forecast, By Indication, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Breast cancer
- 6.3 Non-small cell lung cancer (NSCLC)
- 6.4 Hormone refractory prostate cancer
- 6.5 Gastric adenocarcinoma
- 6.6 Head and neck squamous cell carcinoma (HNSCC)
- 6.7 Other indications
Chapter 7 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Hospitals
- 7.3 Oncology clinics
- 7.4 Other end use
Chapter 8 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 Japan
- 8.4.3 India
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 Alchem International
- 9.2 Alkem Labs
- 9.3 Arch Pharmalabs
- 9.4 Aspen Pharmacare
- 9.5 Cipla
- 9.6 Cisen Pharmaceutical
- 9.7 LGM Pharma
- 9.8 Phyton Biotech
- 9.9 Qilu Pharmaceutical
- 9.10 Teva Active Pharmaceutical Ingredients (TAPI)
- 9.11 Teva Pharmaceuticals
- 9.12 Venus Remedies
- 9.13 Xiromed




