PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1766257

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1766257
Measles, Mumps, Rubella Vaccine Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
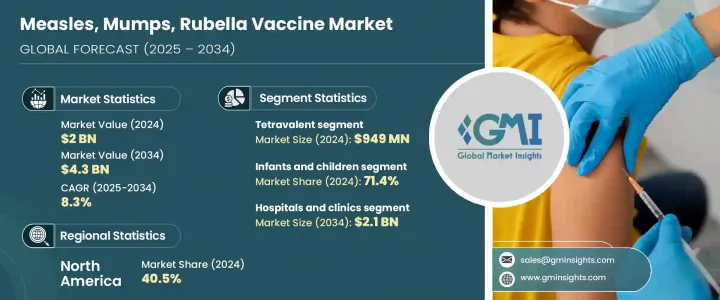
The Global Measles, Mumps, Rubella Vaccine Market was valued at USD 2 billion in 2024 and is estimated to grow at a CAGR of 8.3% to reach USD 4.3 billion by 2034. This expansion can be attributed to several factors, including the growing emphasis on childhood immunization, a rise in outbreaks, and widespread global health initiatives. The issue remains pressing in lower-middle-income nations, where limited resources and inadequate funding necessitate targeted vaccination programs. Furthermore, the increasing government investment in mass vaccination drives aimed at achieving herd immunity is significantly boosting the market, especially in developing regions.
The availability of combination vaccines and thermostable vaccines, which reduce dependence on cold-chain infrastructure, is enhancing vaccine accessibility in remote areas, improving overall efficacy. Rising vaccination awareness, better healthcare infrastructure, and more inclusive national immunization programs are contributing to higher immunization rates globally. Additionally, the increasing birth rates in emerging economies and the integration of MMR vaccines into routine vaccination schedules are expanding the market's target demographic. The ongoing development of enhanced trivalent and quadrivalent vaccines, designed for better disease control, is further supporting market growth.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $2 Billion |
| Forecast Value | $4.3 Billion |
| CAGR | 8.3% |
The tetravalent segment was valued at USD 949 million in 2024. Tetravalent vaccines, which combine multiple vaccinations into one shot, are gaining popularity as they simplify vaccination schedules and improve compliance, particularly in children. These vaccines are now being integrated into national immunization programs worldwide, both in developed and emerging economies, as they offer broader protection and are easier to administer. Their ability to address not only measles and rubella but also varicella, is driving demand for MMRV formulations. Improved cold-chain logistics and support from global health organizations like the WHO are also contributing to the increased adoption of tetravalent vaccines globally, making this segment a key driver of market expansion.
The hospitals and clinics segment is projected to reach USD 2.1 billion by 2034. Hospitals and clinics maintain a dominant role in the MMR vaccine market, largely due to their involvement in immunization campaigns and the range of pediatric services they offer. These healthcare facilities are key centers for vaccine administration in both urban and semi-urban areas, thanks to their well-established cold chain infrastructure and skilled personnel who ensure proper storage and delivery of vaccines. Moreover, during epidemic outbreaks, many nations rely on hospitals as primary hubs for mass immunization programs, further boosting this segment's market share. The expanding number of primary healthcare centers and increasing public-private partnerships in emerging markets also contribute to the growth of this segment.
United States Measles, Mumps, Rubella Vaccine Market was valued at USD 726.1 million in 2024. While the market is mature and heavily regulated, it is still driven by public health recommendations, school immunization mandates, and campaigns responding to localized outbreaks. Although vaccine hesitancy has caused occasional setbacks, pediatric vaccination rates remain high, supported by government funding, controlled pricing, and programs like Vaccines for Children (VFC). The U.S. also continues to play a leading role in vaccine development and exportation, reinforcing its influence on global immunization efforts.
Key strategies adopted by companies in the Global Measles, Mumps, Rubella Vaccine Market include leveraging technological advancements in vaccine delivery, such as the development of combination vaccines and those requiring fewer doses, to increase patient compliance. They also focus on improving accessibility through enhanced cold-chain infrastructure and establishing strong collaborations with global health organizations and governments to ensure vaccines reach underserved regions. Moreover, they invest in research and development to introduce newer, more effective vaccine formulations that provide broader protection against infectious diseases, enhancing both their market presence and long-term sustainability.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional
- 2.2.2 Vaccines type
- 2.2.3 Targeted population
- 2.2.4 End use
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.1.1 Supplier landscape
- 3.1.2 Value addition at each stage
- 3.1.3 Factor affecting the value chain
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising immunization coverage through national vaccination programs
- 3.2.1.2 Increasing pediatric population in developing economies
- 3.2.1.3 Advancements in vaccine formulation and production efficiency
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Stringent regulatory approval processes
- 3.2.2.2 Limited access to vaccines in remote regions
- 3.2.3 Market opportunities
- 3.2.3.1 Expanding public-private partnerships for vaccine distribution
- 3.2.3.2 Growing demand for combination vaccines to reduce injection burden
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.5 Porter's analysis
- 3.6 PESTEL analysis
- 3.7 Technology and innovation landscape
- 3.7.1 Current technological trends
- 3.7.2 Emerging technologies
- 3.8 Price trends
- 3.8.1 By region
- 3.8.2 By vaccines type
- 3.9 Future market trends
- 3.10 Technology and innovation landscape
- 3.10.1 Current technological trends
- 3.10.2 Emerging technologies
- 3.11 Trade statistics (HS code)
- 3.11.1 Major importing countries
- 3.11.2 Major exporting countries
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Mergers and acquisitions
- 4.6.2 Partnerships and collaborations
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Vaccines Type, 2021-2034 ($ Mn)
- 5.1 Key trends
- 5.2 Monovalent
- 5.3 Trivalent (combined MMR)
- 5.4 Tetravalent
Chapter 6 Market Estimates and Forecast, By Targeted Population, 2021-2034 ($ Mn)
- 6.1 Key trends
- 6.2 Infants and children
- 6.3 Adults
Chapter 7 Market Estimates and Forecast, By End Use, 2021-2034 ($ Mn)
- 7.1 Key trends
- 7.2 Hospitals and clinics
- 7.3 Government and public health programs
- 7.4 Other end use
Chapter 8 Market Estimates and Forecast, By Region, 2021-2034 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 Japan
- 8.4.3 India
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 Actiza Pharmaceutical Pvt. Ltd.
- 9.2 Beijing Institute of Biological Products
- 9.3 Biological E
- 9.4 GSK
- 9.5 Merck
- 9.6 Minhai Bio
- 9.7 Sanofi
- 9.8 Serum Institute of India
- 9.9 Shanghai Institute of Biological Products
- 9.10 Sinovac
- 9.11 Zydus Life Science




