PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1766369

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1766369
Epilepsy Treatment Drugs Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
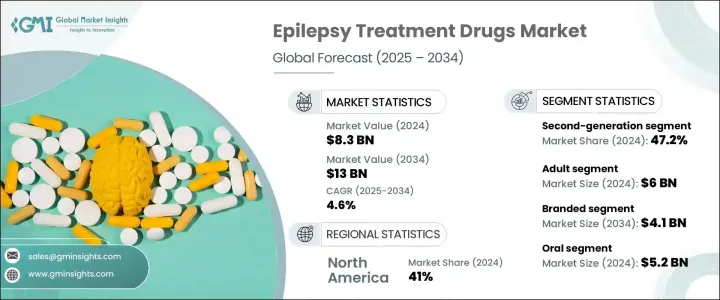
The Global Epilepsy Treatment Drugs Market was valued at USD 8.3 billion in 2024 and is estimated to grow at a CAGR of 4.6% to reach USD 13 billion by 2034. The steady rise in epilepsy diagnoses globally is one of the key factors driving demand for more effective and accessible treatment options. Pharmaceutical innovations, the development of new-age anti-epileptic drugs with fewer side effects, and enhanced treatment adherence are all supporting this upward trend. Growing government and private investments in neurology-related research, alongside increasing awareness and screening rates, are contributing to the expanding market.
The rising global aging population-more vulnerable to neurological disorders-is also expected to further elevate the demand for epilepsy medications. Drug developers are prioritizing therapies that offer better tolerability, sustained release, and improved patient compliance. This shift, paired with improved healthcare infrastructure in developing nations and streamlined regulatory pathways in key markets, is creating a favorable environment for innovation and product adoption. Moreover, the transition toward more personalized medicine strategies, particularly for drug-resistant epilepsy, is improving treatment outcomes and driving long-term market growth.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $8.3 Billion |
| Forecast Value | $13 Billion |
| CAGR | 4.6% |
Anti-epileptic drugs (AEDs), also referred to as anticonvulsants, work by stabilizing irregular electrical signals in the brain, helping to prevent seizures and manage the overall symptoms of epilepsy. Based on drug class, the market is divided into first-generation, second-generation, and third-generation drugs. In 2024, second-generation AEDs accounted for the largest revenue share, contributing 47.2% to the global market. This segment is forecasted to expand at a CAGR of 4.6% from 2025 to 2034. Their broader acceptance is attributed to lower incidences of drug interactions, improved side effect profiles, and better patient adherence compared to older-generation therapies.
In terms of product type, the market is categorized into branded and generic drugs. Branded drugs generated USD 2.6 billion in 2024 and are projected to reach USD 4.1 billion by 2034. Pharmaceutical firms are increasingly focusing on producing branded treatments with improved pharmacokinetics, fewer adverse reactions, and greater therapeutic benefits. The rising need for novel treatments, especially among individuals who do not respond to conventional therapies, is also driving the demand for branded anti-epileptic medications.
When analyzing the route of administration, oral formulations held the largest share in 2024, accounting for USD 5.2 billion. This segment is projected to grow at a CAGR of 4.9% over the forecast period. Oral medications are preferred due to their convenience, ease of use, and the introduction of extended-release versions that improve dosage scheduling and patient adherence. The availability of diverse oral formulations is also accelerating adoption in both developed and emerging markets.
Based on patient demographics, the market is segmented into adult and pediatric populations. The adult segment led with a market value of USD 6 billion in 2024 and is expected to expand at a CAGR of 4.5% through 2034. The increasing incidence of epilepsy among adults is linked to age-related neurological conditions such as strokes, brain injuries, and degenerative disorders. As a result, the demand for reliable and targeted treatment approaches is rising, with a growing emphasis on multi-drug regimens and long-term therapy for improved seizure control.
According to seizure type, the market is segmented into focal seizures, generalized seizures, and combined seizures. The generalized seizure segment generated USD 2.5 billion in 2024 and is estimated to reach USD 4 billion by 2034. A rising number of generalized seizure cases, including tonic-clonic and absence seizures, is prompting the need for more robust therapeutic solutions. This trend is being supported by greater government funding for neurological research, insurance coverage expansions, and policy initiatives aimed at enhancing access to epilepsy care.
In terms of distribution channels, hospital pharmacies, retail pharmacies, and online pharmacies are the major segments. Retail pharmacies captured USD 2.3 billion in revenue in 2024 and are expected to register a CAGR of 4.9% during the forecast period. The growing availability of cost-effective generic drugs in retail settings, especially in low- and middle-income countries, is helping patients manage epilepsy more affordably. Additionally, insurance coverage and subsidy programs are making it easier for patients to access essential medications via retail outlets.
Regionally, North America led the global epilepsy treatment drugs market with a 41% share in 2024. The U.S. alone contributed USD 3.1 billion in revenue that year, driven by high awareness, well-developed healthcare systems, and consistent efforts to improve patient access to advanced neurological treatments. The U.S. market has shown steady year-on-year growth, moving from USD 2.8 billion in 2021 to USD 3 billion in 2023 and reaching USD 3.1 billion in 2024. The increasing prevalence of epilepsy in the region, coupled with favorable regulatory conditions and the presence of global pharmaceutical leaders, continues to push market development.
Major industry players are actively investing in advanced formulations and combination therapies, aiming to enhance patient outcomes and reduce the overall burden of epilepsy on healthcare systems.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Drug class
- 2.2.2 Type
- 2.2.3 Route of administration
- 2.2.4 Age group
- 2.2.5 Seizure type
- 2.2.6 Distribution channel
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising prevalence of epilepsy
- 3.2.1.2 Increasing investments in research and development activities
- 3.2.1.3 Increasing demand for novel treatment for epilepsy
- 3.2.1.4 Growing awareness and early diagnosis
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Adverse effects associated with the antiepileptic drugs
- 3.2.2.2 Patent expiration
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.1.1 U.S. [Food and Drug Administration (FDA)]
- 3.4.1.2 Canada (Health Canada Regulation)
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.4.3.1 Japan (PMDA)
- 3.4.3.2 China (NMPA)
- 3.4.3.3 India (CDSCO)
- 3.4.3.4 Australia (TGA)
- 3.4.1 North America
- 3.5 Pipeline analysis
- 3.6 Future market trends
- 3.7 Gap analysis
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Drug Class, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 First-generation
- 5.3 Second-generation
- 5.4 Third-generation
Chapter 6 Market Estimates and Forecast, By Type, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Branded
- 6.3 Generics
Chapter 7 Market Estimates and Forecast, By Route of Administration, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Oral
- 7.3 Nasal
- 7.4 Injectable
- 7.5 Rectal
Chapter 8 Market Estimates and Forecast, By Age Group, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Pediatric
- 8.3 Adult
Chapter 9 Market Estimates and Forecast, By Seizure Type, 2021 - 2034 ($ Mn)
- 9.1 Key trends
- 9.2 Focal seizure
- 9.3 Generalized seizure
- 9.4 Combined seizure
Chapter 10 Market Estimates and Forecast, By Distribution Channel, 2021 - 2034 ($ Mn)
- 10.1 Key trends
- 10.2 Hospital pharmacies
- 10.3 Retail pharmacies
- 10.4 Online pharmacies
Chapter 11 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 11.1 Key trends
- 11.2 North America
- 11.2.1 U.S.
- 11.2.2 Canada
- 11.3 Europe
- 11.3.1 Germany
- 11.3.2 UK
- 11.3.3 France
- 11.3.4 Spain
- 11.3.5 Italy
- 11.3.6 Netherlands
- 11.4 Asia Pacific
- 11.4.1 China
- 11.4.2 Japan
- 11.4.3 India
- 11.4.4 Australia
- 11.4.5 South Korea
- 11.5 Latin America
- 11.5.1 Brazil
- 11.5.2 Mexico
- 11.5.3 Argentina
- 11.6 Middle East and Africa
- 11.6.1 South Africa
- 11.6.2 Saudi Arabia
- 11.6.3 UAE
Chapter 12 Company Profiles
- 12.1 AbbVie
- 12.2 Bausch Health Companies
- 12.3 Dr. Reddy’s Laboratories
- 12.4 Eisai
- 12.5 GSK
- 12.6 Jazz Pharmaceuticals
- 12.7 Lupin Pharmaceuticals
- 12.8 Neurelis
- 12.9 Novartis
- 12.10 Pfizer
- 12.11 Sanofi
- 12.12 SK Biopharmaceuticals
- 12.13 Sumitomo Pharma
- 12.14 Sun Pharmaceutical Industries
- 12.15 UCB




