PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1876559

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1876559
Personalized Cancer Vaccine Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
The Global Personalized Cancer Vaccine Market was valued at USD 216.9 million in 2024 and is estimated to grow at a CAGR of 26.4% to reach USD 2.2 billion by 2034.
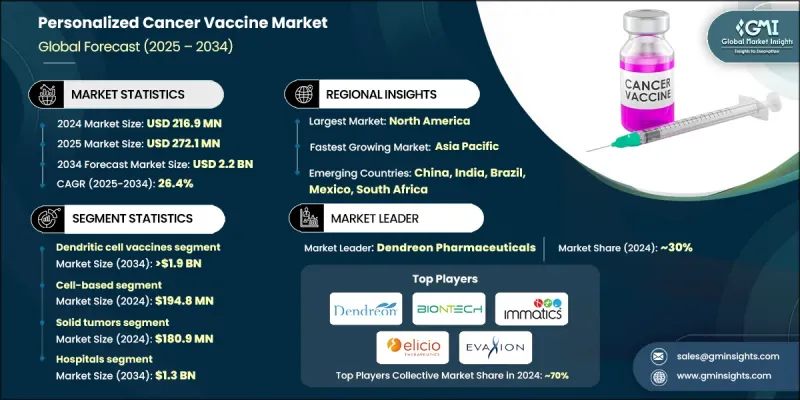
This rapid growth is driven by the increasing prevalence of cancer worldwide, coupled with advancements in genomics, precision medicine, and immunotherapy. Next-generation sequencing (NGS) and genomic profiling allow clinicians to identify tumor-specific neoantigens, which are critical for developing patient-specific vaccines. Declining sequencing costs have made these technologies more accessible in clinical practice. Personalized cancer vaccines train a patient's immune system to recognize and attack their unique cancer cells, offering a targeted alternative to conventional therapies. The rise in cancer cases, aging populations, and growing healthcare expenditure globally are fueling demand, particularly in North America and the Asia-Pacific, where advanced diagnostic and healthcare infrastructure support personalized treatments. These vaccines align with the broader shift toward precision medicine, immunotherapy, and individualized patient care.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $216.9 Million |
| Forecast Value | $2.2 Billion |
| CAGR | 26.4% |
The dendritic cell vaccine segment accounted for an 85.5% share in 2024 and is expected to reach USD 1.9 billion by 2034. Dendritic cells are highly effective antigen-presenting cells capable of eliciting strong T-cell responses, making them ideal for personalized cancer vaccines. DC vaccines provide targeted action, minimize off-target effects, and enhance patient outcomes.
The solid tumors segment generated USD 180.9 million in 2024. Solid tumors are central to more than half of ongoing personalized cancer vaccine trials, including melanoma, non-small cell lung cancer, glioblastoma, and pancreatic cancer. Positive clinical outcomes are accelerating regulatory approvals and driving adoption, validating the efficacy of these vaccines in solid tumor treatment.
The hospitals segment is expected to reach USD 1.3 billion by 2034. Hospitals are the primary sites for vaccine administration, patient monitoring, and clinical trials, positioning them as the most trusted and efficient channels for delivering personalized cancer vaccines. Their role in diagnostics and integrated care makes them essential in patient-centric treatment pathways.
North America Personalized Cancer Vaccine Market held a 46.2% share in 2024. The region hosts nearly half of the global clinical trials for these vaccines, focusing on melanoma, NSCLC, and glioblastoma. Major biotech companies are heavily investing in research and development in North America, supported by public-private partnerships, federal grants, and venture capital funding.
Prominent players in the Global Personalized Cancer Vaccine Market include Immunomic Therapeutics, BioNTech, Elicio Therapeutics, Immatics, Takis Biotech, Candel Therapeutics, VacV Biotherapeutics, Agenus, Moderna, OSE Immunotherapeutics, Evaxion Biotech, Dendreon Pharmaceuticals, ImmunityBio, Infinitopes, and Imugene. Companies in the Personalized Cancer Vaccine Market are implementing multiple strategies to strengthen their market position. They are investing heavily in research and development to enhance vaccine efficacy, optimize antigen selection, and improve patient-specific formulations. Strategic collaborations with academic institutions, hospitals, and biotechnology partners help accelerate clinical validation and regulatory approvals. Firms are expanding geographic footprints through partnerships and licensing agreements to access emerging markets. Additionally, they are leveraging digital tools, AI, and genomic analytics to streamline vaccine design and reduce production timelines.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 360° synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Vaccine type trends
- 2.2.3 Technology area trends
- 2.2.4 Indication trends
- 2.2.5 End use trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.1.1 Supplier landscape
- 3.1.2 Value addition at each stage
- 3.1.3 Factors affecting the value chain
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Advancements in genomics and sequencing technologies
- 3.2.1.2 Increasing integration of artificial intelligence and bioinformatics
- 3.2.1.3 Growing prevalence of cancer and chronic diseases globally
- 3.2.1.4 Increasing shift towards precision medicine
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High production costs
- 3.2.2.2 Complex regulatory landscape
- 3.2.3 Market opportunities
- 3.2.3.1 Expansion into preventive oncology
- 3.2.3.2 Growing pipeline of clinical trials
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.5 Technology landscape
- 3.5.1 Current technological trends
- 3.5.2 Emerging technologies
- 3.6 Future market trends
- 3.7 Pricing analysis
- 3.8 Clinical trial analysis
- 3.9 Porter's analysis
- 3.10 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.2.1 Global
- 4.2.2 North America
- 4.2.3 Europe
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Merger and acquisition
- 4.6.2 Partnership and collaboration
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Vaccine Type, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Dendritic cell vaccines
- 5.3 mRNA-based vaccines
- 5.4 Peptide-based vaccines
- 5.5 Neoantigen-based vaccines
- 5.6 Other vaccine types
Chapter 6 Market Estimates and Forecast, By Technology, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Cell-based
- 6.3 mRNA PCV
- 6.4 Other technologies
Chapter 7 Market Estimates and Forecast, By Indication, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Solid tumors
- 7.3 Hematological malignancies
Chapter 8 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Hospitals
- 8.3 Cancer research centers
- 8.4 Biotechnology and pharmaceutical companies
- 8.5 Other end use
Chapter 9 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 Japan
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 BioNTech
- 10.2 Moderna
- 10.3 Immatics
- 10.4 Candel Therapeutics
- 10.5 Elicio Therapeutics
- 10.6 Evaxion Biotech
- 10.7 Immunomic Therapeutics
- 10.8 Imugene
- 10.9 Infinitopes
- 10.10 OSE Immunotherapeutics
- 10.11 Takis Biotech
- 10.12 VacV Biotherapeutics
- 10.13 ImmunityBio
- 10.14 Agenus
- 10.15 Dendreon Pharmaceuticals




