PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1876646

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1876646
Postpartum Hemorrhage Management Devices Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
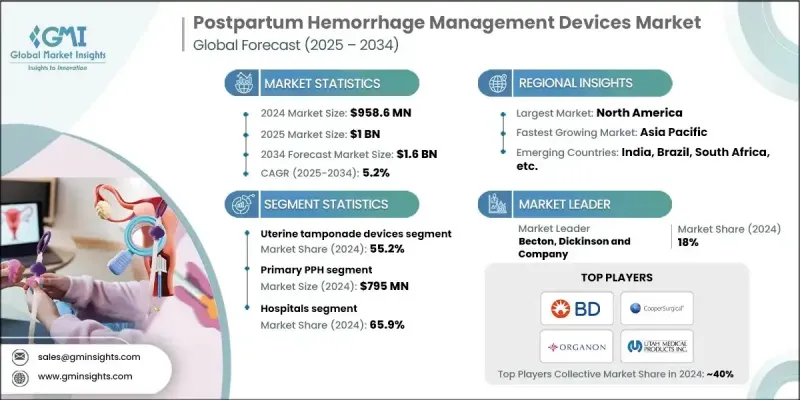
The Global Postpartum Hemorrhage Management Devices Market was valued at USD 958.6 million in 2024 and is estimated to grow at a CAGR of 5.2% to reach USD 1.6 billion by 2034.
The industry continues to advance as the worldwide occurrence of postpartum hemorrhage climbs and awareness of maternal health strengthens. Demand is also influenced by rapid progress in device innovation, growing use of emergency obstetric care, and heightened educational campaigns focused on preventing childbirth complications. These efforts are helping create a more supportive environment for manufacturers by expanding access to maternal care technologies, building stronger collaborations with healthcare systems, and improving overall adoption. Increasing integration of AI-driven analytical tools, digital connectivity, portable equipment, and other technological enhancements is expected to accelerate development over the coming years. Postpartum hemorrhage management devices are designed to control excessive bleeding after childbirth and contribute to reducing maternal mortality during obstetric emergencies. Clinical teams increasingly rely on uterine balloon systems due to their efficiency, minimally invasive nature, and proven effectiveness. Strong safety results and dependable performance continue to broaden their acceptance in healthcare facilities worldwide.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $958.6 Million |
| Forecast Value | $1.6 Billion |
| CAGR | 5.2% |
The uterine tamponade segment held a 55.2% share in 2024, supported by high rates of conditions such as uterine atony and anemia, as well as governmental measures aimed at improving maternal outcomes. These devices deliver internal uterine pressure to manage severe bleeding and can often reduce the need for surgical procedures while stabilizing patients rapidly.
The secondary postpartum hemorrhage segment was valued at USD 163.6 million in 2024. Defined as bleeding occurring more than 24 hours and up to 12 weeks after childbirth, this form of hemorrhage is generally associated with infection, retained tissue, or delayed healing. Diagnostic imaging and treatment using uterotonic medications or surgical options are often required. Although less common than primary postpartum hemorrhage, the secondary category is linked to more complex outcomes and highlights the importance of long-term maternal health planning and preventive-care strategies.
United States Postpartum Hemorrhage Management Devices Market generated USD 330.8 million in 2024. The country's high prevalence of postpartum hemorrhage continues to elevate product demand. The U.S. remains one of the leading adopters of PPH devices due to strong regulatory oversight, a widely developed hospital system, and extensive awareness of maternal health priorities. Public initiatives and private investment in maternal care are broadening access to these devices and stimulating further innovation.
Key Postpartum Hemorrhage Management Devices Market participants include Becton, Dickinson and Company, PREGNA INTERNATIONAL, CooperSurgical, SINAPI, CELOX MEDICAL, ORGANON, STERIMED, ZOEX NIASG, 3rd Stone Design, UTAH MEDICAL PRODUCTS, INC., and MedGyn. Companies competing in the postpartum hemorrhage management devices market are focusing on several strategic actions to enhance their global standing. Many firms are channeling investment toward advanced product engineering to improve reliability, portability, and patient safety. Organizations are expanding R&D programs to introduce new device designs that offer quicker deployment and reduced intervention risks. Strengthening relationships with hospitals and maternal care networks remains a priority, along with broadening distribution in both developed and emerging regions.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 360° synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Product type trends
- 2.2.3 Patient type trends
- 2.2.4 End Use trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising prevalence of postpartum hemorrhage globally
- 3.2.1.2 Increasing maternal mortality awareness campaigns
- 3.2.1.3 Technological advancements in PPH management devices
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Limited access to devices in low-resource settings
- 3.2.2.2 Regulatory delays for new device approvals
- 3.2.3 Opportunities
- 3.2.3.1 Integration of AI for predictive maternal health analytics
- 3.2.3.2 Increased funding for maternal health programs
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.5 Technology and innovation landscape
- 3.5.1 Current technological trends
- 3.5.2 Emerging technologies
- 3.6 Investment landscape
- 3.7 Pricing analysis, 2024
- 3.8 Reimbursement scenario
- 3.9 Sustainability & environmental considerations
- 3.10 Clinical evidence & outcomes analysis
- 3.11 Porter's analysis
- 3.12 PESTEL analysis
- 3.13 Gap analysis
- 3.14 Future market trends
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Company market share analysis
- 4.3.1 Global
- 4.3.2 North America
- 4.3.3 Europe
- 4.3.4 Asia Pacific
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Mergers & acquisitions
- 4.6.2 Partnerships & collaborations
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Product Type, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Uterine tamponade devices
- 5.2.1 Balloon tamponade
- 5.2.2 Vacuum-induced devices
- 5.3 Non-pneumatic anti-shock garments (NASG)
- 5.4 Prefilled injection system
Chapter 6 Market Estimates and Forecast, By Patient Type, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Primary PPH
- 6.3 Secondary PPH
Chapter 7 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Hospitals
- 7.3 Maternity & birthing centers
- 7.4 Ambulatory surgical centers
- 7.5 Other End Use
Chapter 8 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 Japan
- 8.4.3 India
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 MEA
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 3rd Stone Design
- 9.2 Becton, Dickinson and Company
- 9.3 CELOX MEDICAL
- 9.4 CooperSurgical
- 9.5 MedGyn
- 9.6 ORGANON
- 9.7 PREGNA INTERNATIONAL
- 9.8 SINAPI
- 9.9 STERIMED
- 9.10 UTAH MEDICAL PRODUCTS, INC.
- 9.11 ZOEX NIASG




