PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1876655

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1876655
Addisons Disease Drugs Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
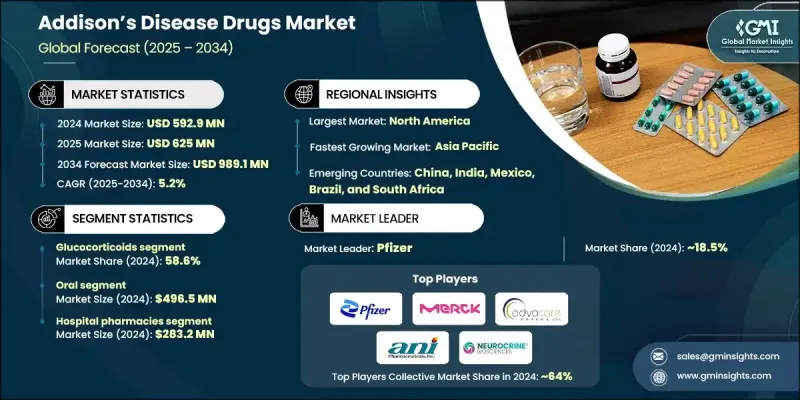
The Global Addisons Disease Drugs Market was valued at USD 592.9 million in 2024 and is estimated to grow at a CAGR of 5.2% to reach USD 989.1 million by 2034.
Growth remains strong as autoimmune disorders tied to adrenal gland failure become more common and healthcare systems expand rare disease programs and awareness initiatives. A rising number of individuals are being diagnosed with primary adrenal insufficiency, which stems from progressive damage to the adrenal cortex and typically requires lifelong corticosteroid replacement. Cases of secondary adrenal insufficiency are also climbing as diagnosis improves for hormonal imbalances caused by pituitary issues or prolonged corticosteroid use. With more patients relying on long-term hormone therapy, demand for dependable treatment options continues to climb. Pharmaceutical manufacturers are responding by enhancing drug delivery systems and refining formulations designed to maintain stable cortisol and aldosterone levels, ultimately improving daily management for those living with chronic adrenal disorders. Addison's disease drugs encompass therapies used to support patients whose adrenal glands are unable to produce adequate essential hormones.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $592.9 Million |
| Forecast Value | $989.1 Million |
| CAGR | 5.2% |
The glucocorticoids category held a 58.6% share in 2024, reflecting its essential role in replacing cortisol in individuals with adrenal insufficiency. Their continued lead is reinforced by broad availability, multiple dosing formats, and strong clinical reliability in helping prevent life-threatening adrenal crises. Advances in extended-release technology and optimized dosing protocols have strengthened adherence and therapeutic outcomes, reinforcing their position in both primary and secondary adrenal insufficiency treatment plans.
The injectable drug segment generated USD 96.4 million in 2024 and is projected to grow at a 4.4% CAGR from 2025 to 2034. Increasing adoption of fast-acting injectable corticosteroids for acute adrenal emergencies is a major contributor to this trend. Greater recognition of self-administration kits and the need for rapid-response medication during severe hormone deficits continues to support demand for injectables across emergency and clinical environments.
North America Addisons Disease Drugs Market held a 40.7% share in 2024. Access to comprehensive hormone replacement therapies across the U.S. and Canada, combined with the presence of established companies such as Pfizer and Merck, bolsters regional growth. Reported cases of adrenal insufficiency associated with autoimmune conditions and chronic infections are increasing, driving higher utilization of available treatments. Supportive rare disease funding and accelerated pathways for emerging drugs, including therapies such as ATRS-1902, contribute to rapid regional expansion. A strong emphasis on personalized drug regimens and digital monitoring tools is expected to enhance adoption across North America.
Leading companies active in the Global Addisons Disease Drugs Market include NEUROCRINE BIOSCIENCES, Hikma, ani Pharmaceuticals, Inc., OMICRON PHARMA, Advacare PHARMA, Merck, Pfizer, Cayman CHEMICAL, GNB PHARMACEUTICALS, and SimSon Pharma Limited. Companies operating in the Addison's disease drugs market apply targeted strategies to expand their competitive reach. Many prioritize advancing hormone replacement therapies through improved formulations that deliver steady hormone levels and reduce dosing complications. Several firms invest in research to refine modified-release corticosteroids and develop innovative delivery systems suited for both chronic management and emergency use. Expanding manufacturing capabilities and optimizing distribution networks help ensure reliable access to life-saving medications. Strategic collaborations with clinical researchers support ongoing trials and broaden therapeutic portfolios.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Drug type trends
- 2.2.3 Route of administration trends
- 2.2.4 Distribution channel trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising prevalence of autoimmune disorders, which are often comorbid with Addison’s disease
- 3.2.1.2 Favourable government support and rare disease funding initiatives
- 3.2.1.3 Advancements in hormone replacement therapies
- 3.2.1.4 Increased awareness about adrenal insufficiency
- 3.2.2 Industry pitfalls & challenges
- 3.2.2.1 Underdiagnosis and misdiagnosis due to non-specific symptoms
- 3.2.2.2 Side effects of long-term corticosteroid use
- 3.2.3 Market opportunities
- 3.2.3.1 Development of sustained-release and transdermal corticosteroids
- 3.2.3.2 Precision medicine and genetic profiling to tailor treatments
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Investment landscape
- 3.5 Pipeline analysis
- 3.6 Future market trends
- 3.7 Reimbursement scenario
- 3.8 Regulatory landscape
- 3.9 Technology landscape
- 3.9.1 Current technologies
- 3.9.2 Emerging technologies
- 3.10 Gap analysis
- 3.11 Porter's analysis
- 3.12 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Company market share analysis
- 4.3.1 North America
- 4.3.2 Europe
- 4.3.3 Asia Pacific
- 4.3.4 LAMEA
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Mergers & acquisitions
- 4.6.2 Partnerships & collaborations
- 4.6.3 New drug launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Drug Type, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Glucocorticoids
- 5.3 Mineralocorticoids
- 5.4 Other drug types
Chapter 6 Market Estimates and Forecast, By Route of Administration, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Oral
- 6.3 Injectable
Chapter 7 Market Estimates and Forecast, By Distribution Channel, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Hospital pharmacies
- 7.3 Retail pharmacies
- 7.4 Online pharmacies
Chapter 8 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 Japan
- 8.4.3 India
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 advacare PHARMA
- 9.2 ani Pharmaceuticals, Inc.
- 9.3 Cayman CHEMICAL
- 9.4 GNB PHARMACEUTICALS
- 9.5 hikma
- 9.6 Merck
- 9.7 NEUROCRINE BIOSCIENCES
- 9.8 OMICRON PHARMA
- 9.9 Pfizer
- 9.10 SimSon Pharma Limited




