PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1885914

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1885914
Healthcare Regulatory Affairs Outsourcing Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
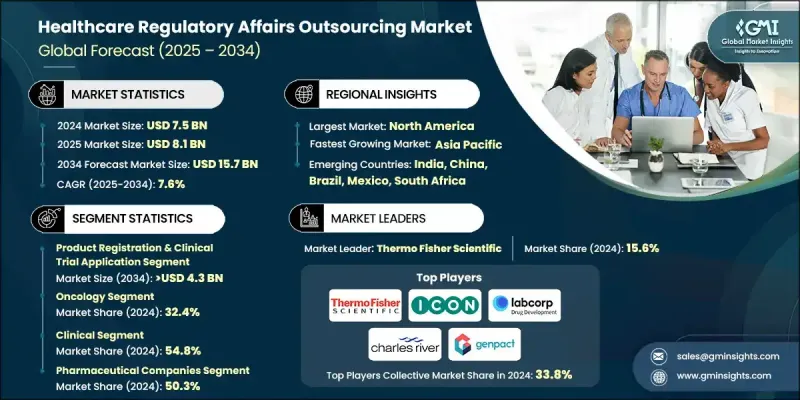
The Global Healthcare Regulatory Affairs Outsourcing Market was valued at USD 7.5 billion in 2024 and is estimated to grow at a CAGR of 7.6% to reach USD 15.7 billion by 2034.
The increasing complexity of global regulatory frameworks compelling pharmaceutical, biotechnology, and medical device companies to outsource compliance, documentation, and submission tasks to specialized partners. The market provides end-to-end solutions that help streamline regulatory processes, ensure adherence to international standards, and accelerate product approvals. Services include regulatory strategy formulation, dossier preparation, eCTD publishing, labeling management, lifecycle maintenance, and regulatory intelligence, all aimed at enhancing efficiency, safety, and patient outcomes. The rapid adoption of digital and cloud-based regulatory information management (RIM) systems, combined with AI-driven analytics, is driving demand for outsourcing providers skilled in managing electronic submissions and complex regulatory workflows. Furthermore, the growing number of global clinical trials, including adaptive and decentralized studies, is increasing regulatory burdens and prompting companies to rely on experienced external partners for submission planning, compliance tracking, and interactions with health authorities.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $7.5 Billion |
| Forecast Value | $15.7 Billion |
| CAGR | 7.6% |
In 2024, the product registration & clinical trial application segment held a 28.4% share. This segment's growth is fueled by increasing drug development activities and stringent regulatory requirements worldwide. Outsourcing is essential to navigate the complexity of submissions, ensuring compliance with agencies like the FDA, EMA, and PMDA, which continue to tighten guidelines to safeguard drug safety and efficacy.
The oncology segment held a 32.4% share in 2024 and is expected to reach USD 5.4 billion during 2025-2034. Rising global cancer incidence, increasing awareness, and early detection programs are driving demand for innovative oncology therapies. Companies are investing heavily in oncology drug development, creating complex, multi-region regulatory submissions. The high cost and long duration of oncology trials further encourage outsourcing to firms with specialized expertise in oncology regulations.
North America Healthcare Regulatory Affairs Outsourcing Market held a 46.5% share in 2024. The region's dominance stems from its robust pharmaceutical, biotechnology, and medical device industries, which require extensive regulatory support to comply with stringent frameworks. Evolving regulations from authorities such as the U.S. FDA and Health Canada, including guidelines on clinical trials, biologics, and post-market monitoring, have increased reliance on outsourcing partners.
Key players operating in the Global Healthcare Regulatory Affairs Outsourcing Market include Thermo Fisher Scientific, Clinilabs, Genpact, ICON, Syneos Health, Accell Clinical Research, Charles River Laboratories, Labcorp, NAMSA, PAREXEL, PharmaLex, ProPharma, and Proventa. Companies in the Healthcare Regulatory Affairs Outsourcing Market strengthen their presence by investing in specialized expertise, particularly for complex therapy areas like oncology and biologics. They develop integrated digital platforms for electronic submissions, AI-driven regulatory intelligence, and cloud-based RIM systems to streamline compliance workflows. Strategic partnerships with global pharmaceutical and biotech firms help expand service portfolios and geographic reach. Firms focus on regulatory consulting, dossier management, and lifecycle maintenance to enhance customer loyalty.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Services trends
- 2.2.3 Indication trends
- 2.2.4 Product stage trends
- 2.2.5 End use trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Growing need to comply with regulatory requirements
- 3.2.1.2 Surging demand for faster approval process for breakthrough drugs and devices
- 3.2.1.3 Rising number of clinical trials
- 3.2.1.4 Increasing regulatory complexity
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Data security and privacy concerns
- 3.2.2.2 Lack of standardization
- 3.2.3 Market opportunities
- 3.2.3.1 Expansion of regulatory outsourcing for emerging markets
- 3.2.3.2 Rising demand for end-to-end RIM
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.4.4 LAMEA
- 3.5 Technology landscape
- 3.5.1 Current technological trends
- 3.5.1.1 Adoption of cloud-based regulatory submission and document management platforms
- 3.5.1.2 Implementation of AI and machine learning for regulatory intelligence and compliance monitoring
- 3.5.1.3 Integration of digital tools for global submission tracking and e-submissions
- 3.5.2 Emerging technologies
- 3.5.2.1 AI-enabled regulatory decision support and predictive compliance analytics
- 3.5.2.2 Blockchain for secure, transparent, and traceable regulatory documentation
- 3.5.1 Current technological trends
- 3.6 Future market trends
- 3.6.1 Regulatory milestone accelerating market entry
- 3.6.2 Increased R&D and investment activity
- 3.6.3 Enhanced regulatory compliance and validation
- 3.7 Investment and funding landscape
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Company market share analysis
- 4.3.1 Global
- 4.3.2 North America
- 4.3.3 Europe
- 4.3.4 Asia Pacific
- 4.4 Competitive positioning matrix
- 4.5 Competitive analysis of major market players
- 4.6 Key developments
- 4.6.1 Mergers & acquisitions
- 4.6.2 Partnerships & collaborations
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Services, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Product registration & clinical trial application
- 5.3 Regulatory consulting/strategic services
- 5.4 Submission management
- 5.5 Legal representation
- 5.6 Regulatory writing & publishing
- 5.7 Other services
Chapter 6 Market Estimates and Forecast, By Indication, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Oncology
- 6.3 Neurology
- 6.4 Cardiology
- 6.5 Immunology
- 6.6 Other indications
Chapter 7 Market Estimates and Forecast, By Product Stage, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Preclinical
- 7.3 Clinical
- 7.4 Post market authorization (PMA)
Chapter 8 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Pharmaceutical companies
- 8.3 Biotechnology companies
- 8.4 Medical device companies
Chapter 9 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 Japan
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Accell Clinical Research
- 10.2 Charles River Laboratories
- 10.3 Clinilabs
- 10.4 Freyr
- 10.5 Genpact
- 10.6 ICON
- 10.7 Labcorp
- 10.8 NAMSA
- 10.9 PAREXEL
- 10.10 PharmaLex
- 10.11 ProPharma
- 10.12 Proventa
- 10.13 Syneos Health
- 10.14 Thermo Fisher Scientific




