PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1892753

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1892753
Exocrine Pancreatic Insufficiency Treatment Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2026 - 2035
The Global Exocrine Pancreatic Insufficiency Treatment Market was valued at USD 3 billion in 2025 and is estimated to grow at a CAGR of 6% to reach USD 5.3 billion by 2035.
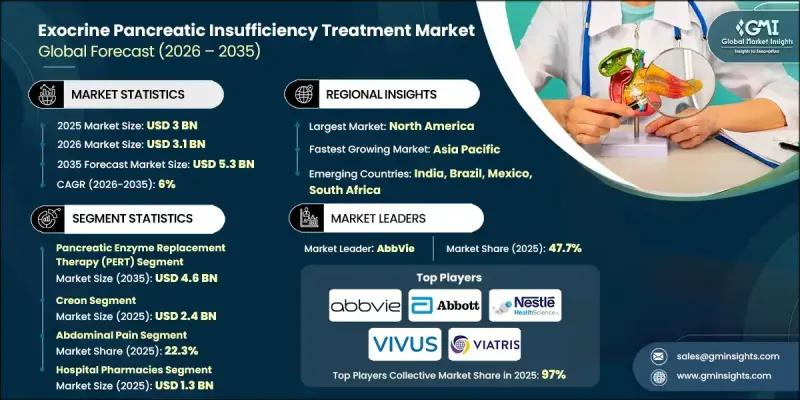
Market growth is fueled by the rising prevalence of chronic pancreatitis, cystic fibrosis, and an aging population, combined with advancements in diagnostic technologies and pharmaceutical innovations. Enhanced diagnostic methods allow earlier detection of EPI, improving treatment outcomes and patient quality of life. Research increasingly focuses on identifying precise biomarkers to assess pancreatic function accurately. Low fecal elastase-1 levels serve as a reliable indicator of pancreatic insufficiency, offering a simpler, outpatient-friendly alternative to invasive procedures. EPI treatment involves medical and dietary management to replace deficient pancreatic enzymes essential for nutrient digestion and absorption. Timely intervention helps reduce symptoms, improve nutrient uptake, and significantly enhance overall patient well-being. The growing awareness of pancreatic disorders and the need for effective treatment solutions is further driving global demand for these therapies.
| Market Scope | |
|---|---|
| Start Year | 2025 |
| Forecast Year | 2026-2035 |
| Start Value | $3 Billion |
| Forecast Value | $5.3 Billion |
| CAGR | 6% |
The pancreatic enzyme replacement therapy (PERT) segment accounted for USD 2.6 billion in 2025 and is projected to reach USD 4.6 billion by 2035. EPI occurs when the pancreas fails to produce or deliver sufficient digestive enzymes, leading to maldigestion and malabsorption. Common underlying conditions include chronic pancreatitis, pancreatic cancer, cystic fibrosis, and diabetes. PERT provides patients with oral administration of pancrelipase, a combination of lipase, protease, and amylase, to restore normal digestive function.
The abdominal pain segment held a 22.3% share in 2025. Abdominal pain is a frequent symptom of EPI, arising from insufficient enzyme production and incomplete digestion. PERT alleviates this discomfort by improving digestion and reducing undigested food accumulation in the intestines. Accurate dosing and timing of enzyme intake with meals are essential for effective symptom control.
U.S. Exocrine Pancreatic Insufficiency Treatment Market was valued at USD 1.62 billion in 2025. Rising incidences of pancreatic disorders, including pancreatic cancer and cystic fibrosis, are primary growth drivers. As the prevalence of these conditions continues to increase, the demand for advanced and effective EPI therapies is expanding, supporting further market growth in North America.
Prominent companies active in the Global Exocrine Pancreatic Insufficiency Treatment Market include Sun Pharmaceutical Industries, Zentiva Pharma, Nordmark Pharma, Abbott Laboratories, Viatris, Digestive Care, Essential Pharma, Eisai, AbbVie, VIVUS, and Nestle. Companies in the EPI treatment sector are enhancing their market presence through targeted product innovation and portfolio expansion. Many are investing in next-generation enzyme formulations and combination therapies to improve efficacy and patient adherence. Research and development initiatives focus on identifying biomarkers and improving diagnostic tools, enabling earlier and more precise treatment. Strategic partnerships with healthcare providers and collaborations with research institutions strengthen credibility and market access. Manufacturers are also leveraging digital platforms and telemedicine solutions to educate patients and support treatment compliance. Expanding geographic presence in high-prevalence regions, optimizing supply chains, and aligning with regulatory standards ensures broader reach and trust.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 360° synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Treatment trends
- 2.2.3 Drug type trends
- 2.2.4 Symptom trends
- 2.2.5 Distribution channel trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing prevalence of chronic pancreatitis (CP) and cystic fibrosis
- 3.2.1.2 Growing aging population
- 3.2.1.3 Advancements in diagnosis
- 3.2.1.4 Technological advancements in pharmaceutical industry
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of treatment
- 3.2.2.2 Limited awareness and education
- 3.2.3 Market opportunities
- 3.2.3.1 Growing demand for personalized medicine
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.5 Future market trends
- 3.6 Pipeline analysis
- 3.7 Porter's analysis
- 3.8 PESTEL analysis
Chapter 4 Competitive Landscape, 2025
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Mergers and acquisitions
- 4.6.2 Partnerships and collaborations
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Treatment, 2022 - 2035 ($ Mn)
- 5.1 Key trends
- 5.2 Nutritional management
- 5.3 Pancreatic enzyme replacement therapy (PERT)
Chapter 6 Market Estimates and Forecast, By Drug Type, 2022 - 2035 ($ Mn)
- 6.1 Key trends
- 6.2 Creon
- 6.3 Zenpep
- 6.4 Pancreaze
- 6.5 Viokace
- 6.6 Other drug types
Chapter 7 Market Estimates and Forecast, By Symptom, 2022 - 2035 ($ Mn)
- 7.1 Key trends
- 7.2 Abdominal pain
- 7.3 Constipation
- 7.4 Diarrhea
- 7.5 Fatty stools
- 7.6 Weight loss
- 7.7 Other symptoms
Chapter 8 Market Estimates and Forecast, By Distribution Channel, 2022 - 2035 ($ Mn)
- 8.1 Key trends
- 8.2 Hospital pharmacies
- 8.3 Retail pharmacies
- 8.4 Online pharmacies
Chapter 9 Market Estimates and Forecast, By Region, 2022 - 2035 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 India
- 9.4.3 Japan
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 Saudi Arabia
- 9.6.2 South Africa
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Abbott Laboratories
- 10.2 AbbVie
- 10.3 Digestive Care
- 10.4 Essential Pharma
- 10.5 Eisai
- 10.6 Nestle
- 10.7 Nordmark Pharma
- 10.8 Sun Pharmaceutical Industries
- 10.9 Viatris
- 10.10 VIVUS
- 10.11 Zentiva Pharma




