PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1892860

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1892860
Optical Coherence Tomography Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
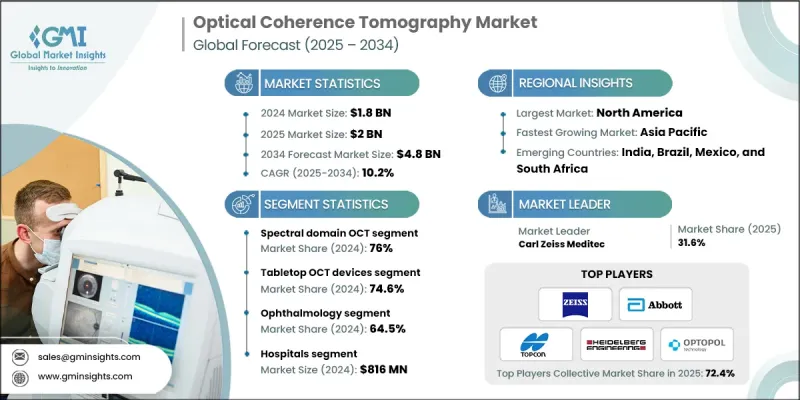
The Global Optical Coherence Tomography Market was valued at USD 1.8 billion in 2024 and is estimated to grow at a CAGR of 10.2% to reach USD 4.8 billion by 2034.
OCT is a non-invasive imaging method that uses low-coherence light to produce highly detailed cross-sectional visuals of biological tissues. The market continues to grow as research and development efforts accelerate the launch of technologies aimed at improving diagnostic clarity, increasing tissue-depth reach, and speeding image acquisition. These advancements support the introduction of next-generation diagnostic tools and therapy-monitoring systems across various clinical environments. Rising demand for compact and portable OCT devices is also influencing development trends, especially as healthcare providers seek accessible solutions for point-of-care testing across rural practices, traveling clinics, home care facilities, and outpatient centers. The growing shift toward mobility and workflow efficiency is encouraging manufacturers to introduce more streamlined and user-friendly imaging systems designed for broader clinical use. Increasing adoption of early-detection strategies for major eye diseases further strengthens the reliance on OCT technology in modern ophthalmic care.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $1.8 Billion |
| Forecast Value | $4.8 Billion |
| CAGR | 10.2% |
The spectral domain OCT segment accounted for a 76% share in 2024. This segment holds a leading position due to rising incidences of chronic eye disorders that impact retinal health and drive the need for precise, fast, and high-resolution imaging. SD-OCT devices are frequently used not only for retinal interpretation but also for assessing the anterior segment, corneal conditions, and the optic nerve. Their adaptability for various clinical tasks, from disease staging to post-treatment evaluations, makes them integral to comprehensive vision assessment.
The tabletop OCT equipment segment held a 74.6% share in 2024. These systems remain the preferred choice for ophthalmic professionals because they offer stable, highly accurate imaging and compatibility with multiple scanning techniques. Their widespread use in eye centers, specialty facilities, and hospital departments is supported by their ability to assist in analyzing glaucoma progression, retinal disorders, and anterior segment health. Modern tabletop devices often combine multiple imaging technologies into a single platform, creating efficient diagnostic workstations.
United States Optical Coherence Tomography Market is projected to reach USD 631 million in 2024 and reach USD 1.6 billion by 2034. Substantial coverage from Medicare and private payers for OCT-related procedures enhances market adoption by lowering costs for both patients and providers. Reimbursement applies to several applications, including optic nerve analysis, retinal scanning, and OCT angiography, which encourages eye care practices to integrate OCT more routinely into clinical workflows.
Key companies participating in the Optical Coherence Tomography Market include Abbott Laboratories, Canon, Agfa-Gevaert, Gentuity, Huvitz, Heidelberg Engineering, Carl Zeiss Meditec, NIDEK, Metall Zug AG (Haag-Streit Group), Moptim (Shenzhen Certainn Technology), Nikon Corporation (Optos plc), Philophos, NotaLVision, NinePoint Medical, Novacam Technologies, OPTOPOL Technology, Topcon Corporation, Tomey, TowardPi (Beijing) Medical Technology, Visionx, Vivolight, YSENMED, and ZD Medical. Key strategies employed by major companies in the Optical Coherence Tomography Market focus on enhancing imaging performance, broadening clinical applications, and expanding product accessibility. Firms continue to invest in advanced light-source technologies and upgraded scanning algorithms to deliver sharper resolution and faster image capture. Many players are integrating multimodal imaging into unified systems to streamline diagnostic processes for clinicians. Collaborations with healthcare providers and academic research centers help refine device accuracy and support clinical validation.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Technology trends
- 2.2.3 Product and services trends
- 2.2.4 Application trends
- 2.2.5 End use trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.1.1 Original equipment manufacturers (OEMs) of OCT components
- 3.1.2 Manufacturer
- 3.1.3 Regulatory authorities
- 3.1.4 Distributors and suppliers
- 3.1.5 End use
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Growing prevalence of eye disorders
- 3.2.1.2 Advancements in OCT technology
- 3.2.1.3 Rising adoption of non-invasive diagnostic techniques
- 3.2.1.4 Increasing healthcare investments and awareness in emerging economies
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of therapies
- 3.2.2.2 Shortage of skilled professionals trained to operate and interpret OCT systems
- 3.2.3 Market opportunities
- 3.2.3.1 AI-powered diagnostic algorithms and automated analysis
- 3.2.3.2 Increasing applications of OTC in pediatric and critical care
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 U.S.
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.5 Technology landscape
- 3.5.1 Current technological trends
- 3.5.1.1 Swept source OCT for deep tissue visualization
- 3.5.1.2 OCT angiography for non-invasive vascular mapping
- 3.5.1.3 Portable and multimodal oct devices for point-of-care diagnostics
- 3.5.2 Emerging technologies
- 3.5.2.1 Quantum-enhanced OCT for ultra-sensitive imaging
- 3.5.2.2 Adaptive optics integration for real-time aberration correction
- 3.5.2. 3. Ultra-high-speed OCT for dynamic 3D tissue visualization
- 3.5.1 Current technological trends
- 3.6 Patent analysis
- 3.6.1 Key patent holders and technology leaders
- 3.6.2 Patent expiration analysis and impact
- 3.6.3 Patent litigation and disputes
- 3.6.4 Geographic patent protection strategies
- 3.7 Pricing analysis, 2024
- 3.8 Future market trends
- 3.8.1 AI-driven OCT platforms for predictive diagnostics
- 3.8.2 Cloud-based OCT data management and telemedicine integration
- 3.8.3 Expansion of OCT applications beyond ophthalmology
- 3.9 Supply chain and distribution analysis
- 3.9.1 Raw material sourcing
- 3.9.2 Manufacturing hub analysis
- 3.9.3 Distribution channel mapping and partner networks
- 3.9.4 Supply chain vulnerabilities and risk mitigation
- 3.10 Porter's analysis
- 3.11 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.2.1 Global
- 4.2.2 North America
- 4.2.3 Europe
- 4.2.4 Asia Pacific
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key development
Chapter 5 Market Estimates and Forecast, By Technology, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Spectral domain OCT
- 5.3 Swept-source OCT
- 5.4 Time domain OCT
Chapter 6 Market Estimates and Forecast, By Product and Services, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Instruments
- 6.2.1 Tabletop OCT devices
- 6.2.2 Catheter based OCT devices
- 6.2.3 Handheld OCT devices
- 6.2.4 Doppler OCT devices
- 6.3 Component replacement services and software licensing and upgrade
- 6.4 Other services
Chapter 7 Market Estimates and Forecast, By Application, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Ophthalmology
- 7.3 Cardiology
- 7.4 Dermatology
- 7.5 Oncology
- 7.6 Other applications
Chapter 8 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Hospitals
- 8.3 Diagnostic imaging centers
- 8.4 Ambulatory surgical centers
- 8.5 Other end use
Chapter 9 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Italy
- 9.3.5 Spain
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 India
- 9.4.3 Japan
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 Saudi Arabia
- 9.6.2 South Africa
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Abbott Laboratories
- 10.2 Agfa-Gevaert
- 10.3 Canon
- 10.4 Carl Zeiss Meditec
- 10.5 Gentuity
- 10.6 Heidelberg Engineering
- 10.7 Huvitz
- 10.8 Metall Zug AG (Haag-Streit Group)
- 10.9 Moptim (Shenzhen Certainn Technology)
- 10.10 NIDEK
- 10.11 Nikon Corporation (Optos plc)
- 10.12 NinePoint Medical
- 10.13 Notal Vision
- 10.14 Novacam Technologies
- 10.15 OPTOPOL Technology
- 10.16 Philophos
- 10.17 Tomey
- 10.18 Topcon Corporation
- 10.19 TowardPi (Beijing) Medical Technology
- 10.20 Visionx
- 10.21 Vivolight
- 10.22 YSENMED
- 10.23 ZD medical




