PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1892872

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1892872
Home Sleep Apnea Testing Devices Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2026 - 2035
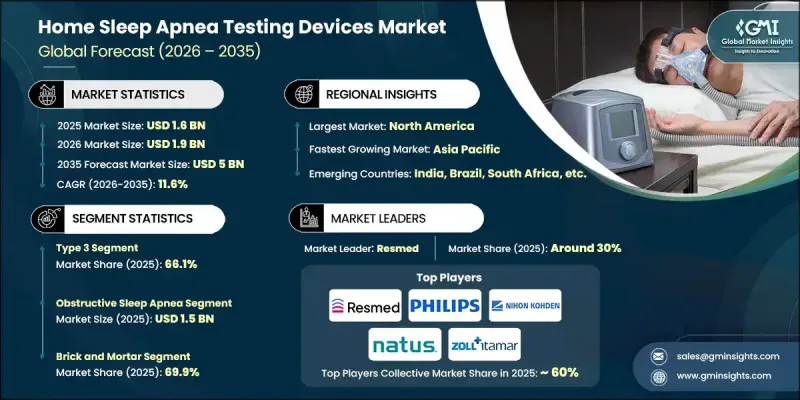
The Global Home Sleep Apnea Testing Devices Market was valued at USD 1.6 billion in 2025 and is estimated to grow at a CAGR of 11.6% to reach USD 5 billion by 2035.
Market growth is fueled by the expanding elderly population, rising incidence of sleep apnea and related chronic conditions, and rapid innovation in portable and wearable diagnostic technologies. Increasing public awareness of sleep health and the clinical risks associated with undiagnosed sleep disorders is further supporting demand. Home-based testing solutions are gaining preference due to their affordability, convenience, and ability to deliver reliable diagnostic results outside traditional clinical environments. The growing adoption of telemedicine and remote patient monitoring is also accelerating uptake, as these devices integrate easily into digital healthcare workflows. The rising prevalence of obesity, diabetes, and cardiovascular disease is increasing the need for early screening and intervention, since untreated sleep apnea significantly elevates the risk of serious complications. Advances in sensor accuracy, wireless data transmission, and artificial intelligence are enabling devices that are smaller, more precise, and simpler for patients to operate independently. These factors are positioning home sleep apnea testing as a practical frontline diagnostic tool across global healthcare systems.
| Market Scope | |
|---|---|
| Start Year | 2025 |
| Forecast Year | 2026-2035 |
| Start Value | $1.6 Billion |
| Forecast Value | $5 Billion |
| CAGR | 11.6% |
The type 3 device segment held a 66.1% share in 2025. Growth in this segment is supported by increased emphasis on early diagnosis and preventive care. These devices track multiple physiological signals and offer a balance between clinical reliability and user convenience, making them suitable for identifying moderate to severe obstructive conditions in uncomplicated cases.
The obstructive sleep apnea segment was valued at USD 1.5 billion in 2025. This condition represents the most common form of sleep apnea and is closely associated with metabolic and cardiovascular risks, reinforcing the importance of timely diagnosis and monitoring.
North America Home Sleep Apnea Testing Devices Market accounted for a 33.6% share in 2025 and is expected to maintain strong growth. High awareness of sleep disorders, widespread telehealth adoption, advanced healthcare infrastructure, and elevated prevalence of lifestyle-related risk factors continue to drive regional demand.
Key companies active in the Global Home Sleep Apnea Testing Devices Market include ResMed, Philips, Nox Medical, Compumedics, ZOLL Itamar, Nihon Kohden, Natus, SOMNOmedics, CleveMed, Neurosoft, CADWELL, CONTEC, NEUROVIRTUAL, and Nox Medical. Companies in the Global Home Sleep Apnea Testing Devices Market are strengthening their market position through continuous product innovation, digital integration, and strategic partnerships. Manufacturers focus on improving diagnostic accuracy, comfort, and ease of use through advanced sensors and AI-driven analytics. Integration with telehealth platforms and cloud-based data management systems supports seamless clinical workflows. Many players invest in regulatory approvals and clinical validation to expand adoption across healthcare settings. Geographic expansion into underserved markets and collaboration with sleep clinics and healthcare providers help increase reach.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 360° synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Test type trends
- 2.2.3 Indication trends
- 2.2.4 Distribution channel trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Growing prevalence of sleep apnea and related co-morbidities
- 3.2.1.2 Increasing awareness regarding sleep health and home sleep apnea tests
- 3.2.1.3 Rising aging population
- 3.2.1.4 Technological advancements in wearable and portable HSAT devices
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Stringent regulatory approvals for diagnostic devices
- 3.2.2.2 Limited accuracy compared to in-lab polysomnography
- 3.2.3 Opportunities
- 3.2.3.1 Development of AI-powered diagnostic algorithms
- 3.2.3.2 Growth of telehealth platforms offering HSAT services
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.4.4 LAMEA
- 3.5 Technology and innovation landscape
- 3.5.1 Current technological trends
- 3.5.2 Emerging technologies
- 3.6 Value chain analysis
- 3.7 Reimbursement scenario
- 3.8 Policy landscape
- 3.9 Consumer behavior insights
- 3.10 Porter's analysis
- 3.11 PESTEL analysis
- 3.12 Gap analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Company market share analysis
- 4.3.1 Global
- 4.3.2 North America
- 4.3.3 Europe
- 4.3.4 Asia Pacific
- 4.3.5 LAMEA
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Mergers & acquisitions
- 4.6.2 Partnerships & collaborations
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Test Type, 2022 - 2035 ($ Mn)
- 5.1 Key trends
- 5.2 Type 2
- 5.3 Type 3
- 5.4 Type 4
Chapter 6 Market Estimates and Forecast, By Application, 2022 - 2035 ($ Mn)
- 6.1 Key trends
- 6.2 Obstructive sleep apnea (OSA)
- 6.3 Central sleep apnea
Chapter 7 Market Estimates and Forecast, By Distribution Channel, 2022 - 2035 ($ Mn)
- 7.1 Key trends
- 7.2 Brick and mortar
- 7.3 E-commerce
Chapter 8 Market Estimates and Forecast, By Region, 2022 - 2035 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 Japan
- 8.4.3 India
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 MEA
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 CADWELL
- 9.2 CleveMed
- 9.3 COMPUMEDICS
- 9.4 CONTEC
- 9.5 natus
- 9.6 Neurosoft
- 9.7 NEUROVIRTUAL
- 9.8 NIHON KOHDEN
- 9.9 nox MEDICAL
- 9.10 PHILIPS
- 9.11 Resmed
- 9.12 SOMNOmedics
- 9.13 ZOLL itamar




