PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1913443

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1913443
U.S. Ophthalmic Sutures Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2026 - 2035
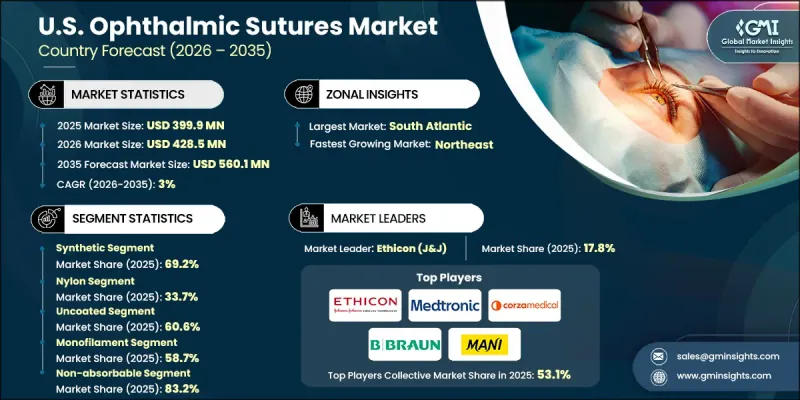
U.S. Ophthalmic Sutures Market was valued at USD 399.9 million in 2025 and is estimated to grow at a CAGR of 3% to reach USD 560.1 million by 2035.
The growth is fueled by rising demand for advanced ophthalmic surgeries, an increase in the prevalence of eye disorders, and the expanding adoption of minimally invasive procedures. Ophthalmic sutures are vital for hospitals, specialized eye-care centers, and ambulatory surgical facilities, ensuring precise wound closure, improved surgical outcomes, and greater efficiency in procedures involving cataract, corneal, retinal, and glaucoma interventions. The market is further supported by a growing volume of surgeries, technological advancements in absorbable and non-absorbable sutures, and a focus on materials designed for optimal handling, reduced tissue reactivity, and predictable postoperative healing. Increasing surgeon awareness and the shift toward higher-quality surgical solutions are also driving market expansion across the U.S. as the population ages and chronic ocular conditions rise.
| Market Scope | |
|---|---|
| Start Year | 2025 |
| Forecast Year | 2026-2035 |
| Start Value | $399.9 Million |
| Forecast Value | $560.1 Million |
| CAGR | 3% |
The synthetic sutures segment held 69.2% share in 2025, due to high demand for durable, biocompatible, and minimally reactive materials. Surgeons favor synthetic sutures for their predictable absorption rates and reduced risk of postoperative complications, especially in delicate procedures such as cataract surgeries, corneal transplants, and vitreoretinal interventions.
The coated sutures segment generated USD 157.5 million in 2025 and is expected to grow at a CAGR of 2.8% through 2035. Coated sutures, enhanced with protective layers such as silicone or polyglactin, facilitate smoother passage through ocular tissues, minimize trauma, and accelerate wound healing, supporting high-precision surgeries.
South Atlantic Ophthalmic Sutures Market held a 19.4% share in 2025. States like Florida, Georgia, North Carolina, and South Carolina have a large aging population, driving higher ophthalmic surgery volumes. Well-established hospitals and specialty clinics with advanced surgical infrastructure further support the adoption of premium sutures.
Key players in the U.S. Ophthalmic Sutures Market include Accutome, Alcon, Assut Medical, Aurolab, B. Braun, Corza Medical, DemeTECH, Ethicon, FCI Ophthalmics, Geuder AG, Mani, Medtronic, Peters Surgical, Rumex International, Surgical Specialties Corporation, and Teleflex Incorporated. Companies in the U.S. Ophthalmic Sutures Market are strengthening their position through strategic collaborations with hospitals and surgeons, expanding product portfolios to include advanced synthetic and coated sutures, and investing in R&D to improve handling, absorption, and biocompatibility. Many are focusing on minimally invasive procedure compatibility and enhanced postoperative outcomes to differentiate their offerings. Marketing initiatives, surgeon training programs, and global regulatory compliance further enhance brand credibility.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Zonal
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Zonal trends
- 2.2.2 Type trends
- 2.2.3 Material trends
- 2.2.4 Coating trends
- 2.2.5 Material structure trends
- 2.2.6 Absorption trends
- 2.2.7 Application trends
- 2.2.8 End use trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 High burden of chronic eye diseases in the U.S.
- 3.2.1.2 Strong penetration of advanced ophthalmic surgical technologies
- 3.2.1.3 High diabetes prevalence leading to complex ophthalmic complications
- 3.2.1.4 Expansion of outpatient eye surgery centers (ASCs)
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Rising procedure costs and reimbursement pressures
- 3.2.2.2 Shortage of ophthalmologists and retina specialists
- 3.2.3 Market opportunities
- 3.2.3.1 Growing adoption of premium, specialty, and biodegradable sutures
- 3.2.3.2 Integration of advanced eye-care networks and expansion of surgical capacity
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technological advancements
- 3.5.1 Current technological trends
- 3.5.1.1 Microsurgical minimally invasive techniques
- 3.5.1.2 High-precision cataract sutures
- 3.5.1.3 Streamlined operating room workflow
- 3.5.2 Emerging technologies
- 3.5.2.1 Composite ophthalmic suture solutions
- 3.5.2.2 Robotic-assisted suturing tools
- 3.5.2.3 Smart surgical instruments
- 3.5.1 Current technological trends
- 3.6 Gap analysis
- 3.7 Porter's analysis
- 3.8 PESTEL analysis
- 3.9 Future market trends
- 3.9.1 Robotic-assisted ophthalmic procedures
- 3.9.2 Bioengineered advanced suture materials
- 3.9.3 Drug-eluting ophthalmic sutures
Chapter 4 Competitive Landscape, 2025
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Company market share analysis
- 4.4 Competitive positioning matrix
- 4.5 Competitive analysis of major market players
- 4.6 Key developments
- 4.6.1 Mergers & acquisitions
- 4.6.2 Partnerships & collaborations
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Type, 2022 - 2035 ($ Mn)
- 5.1 Key trends
- 5.2 Natural
- 5.3 Synthetic
Chapter 6 Market Estimates and Forecast, By Material, 2022 - 2035 ($ Mn)
- 6.1 Key trends
- 6.2 PGA
- 6.3 Nylon
- 6.4 Silk
- 6.5 Polypropylene
- 6.6 Other materials
Chapter 7 Market Estimates and Forecast, By Coating, 2022 - 2035 ($ Mn)
- 7.1 Key trends
- 7.2 Coated
- 7.3 Uncoated
Chapter 8 Market Estimates and Forecast, By Material Structure, 2022 - 2035 ($ Mn)
- 8.1 Key trends
- 8.2 Monofilament
- 8.3 Multifilament/Braided
Chapter 9 Market Estimates and Forecast, By Absorption, 2022 - 2035 ($ Mn)
- 9.1 Key trends
- 9.2 Absorbable
- 9.3 Non-absorbable
Chapter 10 Market Estimates and Forecast, By Application, 2022 - 2035 ($ Mn)
- 10.1 Key trends
- 10.2 Cataract surgery
- 10.3 Corneal transplantation surgery
- 10.4 Glaucoma surgery
- 10.5 Vitrectomy
- 10.6 Oculoplastic surgery
- 10.7 Other applications
Chapter 11 Market Estimates and Forecast, By End Use, 2022 - 2035 ($ Mn)
- 11.1 Key trends
- 11.2 Hospitals
- 11.3 Ambulatory surgical centers
- 11.4 Other end use
Chapter 12 Market Estimates and Forecast, By Zone, 2022 - 2035 ($ Mn)
- 12.1 Key trends
- 12.2 East North Central
- 12.2.1 Illinois
- 12.2.2 Indiana
- 12.2.3 Michigan
- 12.2.4 Ohio
- 12.2.5 Wisconsin
- 12.3 West South Central
- 12.3.1 Arkansas
- 12.3.2 Louisiana
- 12.3.3 Oklahoma
- 12.3.4 Texas
- 12.4 South Atlantic
- 12.4.1 Delaware
- 12.4.2 Florida
- 12.4.3 Georgia
- 12.4.4 Maryland
- 12.4.5 North Carolina
- 12.4.6 South Carolina
- 12.4.7 Virginia
- 12.4.8 West Virginia
- 12.4.9 Washington, D.C.
- 12.5 Northeast
- 12.5.1 Connecticut
- 12.5.2 Maine
- 12.5.3 Massachusetts
- 12.5.4 New Hampshire
- 12.5.5 Rhode Island
- 12.5.6 Vermont
- 12.5.7 New Jersey
- 12.5.8 New York
- 12.5.9 Pennsylvania
- 12.6 East South Central
- 12.6.1 Alabama
- 12.6.2 Kentucky
- 12.6.3 Mississippi
- 12.6.4 Tennessee
- 12.7 West North Central
- 12.7.1 Iowa
- 12.7.2 Kansas
- 12.7.3 Minnesota
- 12.7.4 Missouri
- 12.7.5 Nebraska
- 12.7.6 North Dakota
- 12.7.7 South Dakota
- 12.8 Pacific
- 12.8.1 Alaska
- 12.8.2 California
- 12.8.3 Hawaii
- 12.8.4 Oregon
- 12.8.5 Washington
- 12.9 Mountain States
- 12.9.1 Arizona
- 12.9.2 Colorado
- 12.9.3 Utah
- 12.9.4 Nevada
- 12.9.5 New Mexico
- 12.9.6 Idaho
- 12.9.7 Montana
- 12.9.8 Wyoming
Chapter 13 Company Profiles
- 13.1 Accutome
- 13.2 Alcon
- 13.3 Assut Medical
- 13.4 Aurolab
- 13.5 B. Braun
- 13.6 Corza Medical
- 13.7 DemeTECH
- 13.8 Ethicon
- 13.9 FCI Ophthalmics
- 13.10 Geuder AG
- 13.11 Mani
- 13.12 Medtronic
- 13.13 Peters Surgical
- 13.14 Rumex International
- 13.15 Surgical Specialties Corporation
- 13.16 Teleflex Incorporated




