PUBLISHER: MarketsandMarkets | PRODUCT CODE: 1891772

PUBLISHER: MarketsandMarkets | PRODUCT CODE: 1891772
Preimplantation Genetic Testing Market by Product & Service (Reagents, Consumables, Instruments, Software), Technology (NGS, PCR, SNP, CGH), Procedure (Screening, Diagnosis), Application (Aneuploidy, HLA Typing), Type of cycle - Global Forecast to 2030
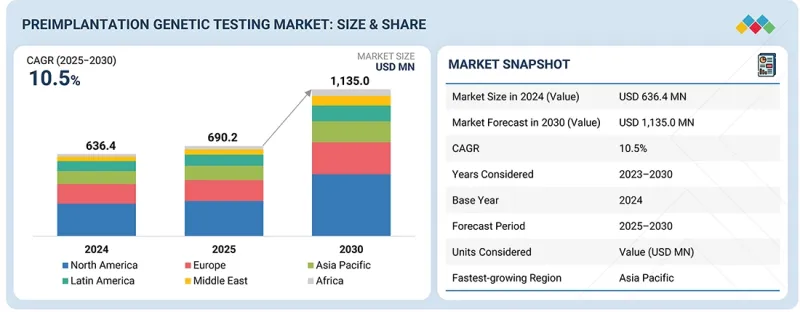
The preimplantation genetic testing market is expected to reach USD 1,135.0 million by 2030, up from USD 690.2 million in 2025, at a CAGR of 10.5% during the forecast period. The market growth is primarily driven by the increasing prevalence of genetic disorders, growing awareness regarding early genetic diagnosis, and the rising adoption of in-vitro fertilization (IVF) procedures worldwide.
| Scope of the Report | |
|---|---|
| Years Considered for the Study | 2024-2033 |
| Base Year | 2024 |
| Forecast Period | 2025-2030 |
| Units Considered | Value (USD million) |
| Segments | Procedure Type, Technology, Product & Service, Application, Type of Cycle, End User |
| Regions covered | North America, Europe, the Asia Pacific, Latin America, the Middle East, and Africa |
The demand for preimplantation genetic testing is also supported by advancements in next-generation sequencing (NGS) and polymerase chain reaction (PCR) technologies that enhance testing accuracy and efficiency. Moreover, the growing trend toward delayed pregnancies and the rising risk of chromosomal abnormalities in embryos are contributing to the market expansion. However, the high costs associated with IVF and genetic testing procedures, along with ethical and regulatory challenges, continue to hinder widespread adoption.
"The aneuploidy application segment is expected to grow at the highest CAGR during the forecast period."
The aneuploidy application segment is projected to grow at the highest CAGR in the preimplantation genetic testing market during the forecast period. This growth is primarily driven by the rising incidence of chromosomal abnormalities, particularly among women of advanced maternal age, and the increasing demand for accurate embryo screening in IVF procedures. Aneuploidy enables the detection of abnormal chromosome numbers, helping improve implantation rates and reduce the risk of miscarriage. The integration of advanced genomic tools such as next-generation sequencing (NGS) and array-based comparative genomic hybridization (aCGH) has further enhanced test precision and efficiency. Additionally, growing awareness of genetic screening benefits and the rising adoption of assisted reproductive technologies are contributing to the strong growth of the aneuploidy segment globally.
"The reagents and consumables segment holds the largest share of the market."
The reagents and consumables segment holds the largest share of the preimplantation genetic testing market. This dominance is due to their repeated use at every stage of the testing process, including sample preparation, amplification, and analysis. The increasing number of IVF procedures and genetic tests has greatly boosted the demand for high-quality reagents, assay kits, and consumables that provide accuracy, reproducibility, and consistent results. Additionally, advances in next-generation sequencing (NGS), polymerase chain reaction (PCR), and array-based platforms have enabled the development of specialized reagents for high-throughput and precise embryo analysis. Ongoing product innovations by major market players, along with the growing use of genetic screening in fertility clinics and research labs, further drive segment growth. The essential and recurring nature of these products secures their leading role in the global preimplantation genetic testing market.
"The US is expected to grow at the highest CAGR during the forecast period."
The US is expected to have the highest growth rate in the preimplantation genetic testing market, driven by several key factors. The strong presence of leading IVF clinics, advanced genomic labs, and a well-established healthcare system significantly contributes to market growth in the country. Additionally, the increasing rate of infertility, rising average maternal age, and growing awareness of genetic screening options are boosting demand for preimplantation genetic testing in the US. Favorable reimbursement policies, supportive government initiatives for reproductive health, and ongoing investments in genomic research further drive market expansion.
Furthermore, the growing integration of next-generation sequencing (NGS) and polymerase chain reaction (PCR) technologies in IVF procedures is improving testing accuracy and efficiency. Increasing access to assisted reproductive technologies, along with the rising preference for personalized reproductive care, continues to drive the adoption of preimplantation genetic testing across the region. The US's robust regulatory framework ensures test reliability and clinical validity, bolstering confidence in preimplantation genetic testing solutions and reinforcing North America's leading position in the global market.
The primary interviews conducted for this report can be categorized as follows:
- By Respondent: Tier 1 - 25%, Tier 2 - 35%, and Tier 3 - 40%
- By Designation: Managers - 45%, CXOs and Directors - 30%, and Executives - 25%
- By Region: North America - 35%, Europe - 25%, Asia Pacific - 15%, Latin America - 10%, the Middle East - 10%, and Africa - 5%
Illumina, Inc. (US), Thermo Fisher Scientific Inc. (US), Agilent Technologies, Inc. (US), Revvity (US), CooperCompanies (US), Abbott (US), Takara Bio Inc. (Japan), QIAGEN (Germany), Vitrolife (Sweden), Oxford Nanopore Technologies plc (UK), Oxford Gene Technology IP Limited (UK), Yikon Genomics (China), Shiva Scientific (India), Nanjing Superyears Gene Technology Co. Ltd. (China), Medicover Genetics (Germany), MedGenome (India), Fulgent Genetics (US), Invicta (Poland), Genea Pty Limited (Australia), SciGene Corporation (US), Bioarray S.L. (Spain), Unimed Biotech (Shanghai) Co., Ltd. (China), GeneMind Biosciences Co. Ltd. (China), Berry Genomics (China), and Bangkok Genomics Innovation (Thailand) are some of the key companies offering preimplantation genetic testing products.
Research Coverage
This research report categorizes the preimplantation genetic testing market by procedure type (preimplantation genetic screening, preimplantation genetic diagnosis), technology (next-generation sequencing, polymerase chain reaction, fluorescence in situ hybridization, comparative genomic hybridization, single-nucleotide polymorphism), product & service (reagents & consumables, instruments, software & services), application (aneuploidy, structural chromosomal abnormalities [translocations, deletions, duplications, inversions], single gene disorders, X-linked disorders, HLA typing, gender identification), type of cycle (fresh non-donor, frozen non-donor, fresh donor, frozen donor), end user (fertility clinics, hospitals, diagnostic laboratories, other end users), and region (North America, Europe, Asia Pacific, Latin America, Middle East, And Africa).
The report's scope encompasses detailed information about the primary factors, including drivers, restraints, challenges, and opportunities, that influence the growth of the preimplantation genetic testing market. A comprehensive analysis of key industry players has been performed to provide insights into their business overview, product portfolio, key strategies, new product launches, acquisitions, and recent developments related to the preimplantation genetic testing market. This report also includes a competitive analysis of emerging startups in the preimplantation genetic testing industry ecosystem.
Key Benefits of Buying the Report
The report will assist market leaders and new entrants by providing revenue estimates for the overall market and its subsegments. It will also help stakeholders better understand the competitive landscape and gain more insights to position their businesses effectively and develop suitable go-to-market strategies. This report will enable stakeholders to grasp the market's pulse and offer information on key market drivers, restraints, opportunities, and challenges.
The report provides insights into the following pointers:
- Analysis of key drivers (decline in fertility, rise of fertility tourism in emerging economies, increasing number of fertility clinics and IVF centers, increasing public-private investments to develop novel diagnostic techniques, and high risk of chromosomal abnormalities with advancing maternal age), restraints (high procedural cost in preimplantation genetic testing and unfavorable government regulations and healthcare reforms for IVF procedures), opportunities (improving healthcare infrastructure and rising medical tourism in emerging economies and use of fertility treatments by single parents and same-sex couples), and challenges (socio-ethical concerns surrounding preimplantation genetic testing and procedural limitations with advancing age) influencing the market growth
- Product Development/Innovation: Detailed insights into newly launched products and technological assessment of the preimplantation genetic testing market
- Market Development: Comprehensive information about lucrative markets and analysis of the market across varied regions
- Market Diversification: Exhaustive information about new products, untapped geographies, recent developments, and investments in the preimplantation genetic testing market
- Competitive Assessment: In-depth assessment of market shares, growth strategies, and product offerings of leading players, including Illumina, Inc. (US), Thermo Fisher Scientific Inc. (US), and Agilent Technologies, Inc. (US), Revvity (US), CooperCompanies (US), and Abbott (US), among others offering products and services for preimplantation genetic testing market. Other companies include MedGenome (India), Fulgent Genetics (US), Invicta (Poland), Genea Pty Limited (Australia), SciGene Corporation (US), Bioarray S.L. (Spain), Unimed Biotech (Shanghai) Co., Ltd (China), and GeneMind Biosciences Co., Ltd. (China), among others, for the preimplantation genetic testing market
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 STUDY OBJECTIVES
- 1.2 MARKET DEFINITION
- 1.3 STUDY SCOPE
- 1.3.1 MARKET SEGMENTATION & REGIONAL SCOPE
- 1.3.2 INCLUSIONS & EXCLUSIONS
- 1.3.3 YEARS CONSIDERED
- 1.3.4 CURRENCY CONSIDERED
- 1.4 STAKEHOLDERS
- 1.5 SUMMARY OF CHANGES
2 EXECUTIVE SUMMARY
- 2.1 KEY INSIGHTS & MARKET HIGHLIGHTS
- 2.2 KEY MARKET PARTICIPANTS: INSIGHTS & STRATEGIC DEVELOPMENTS
- 2.3 DISRUPTIVE TRENDS SHAPING PREIMPLANTATION GENETIC TESTING MARKET
- 2.4 HIGH-GROWTH SEGMENTS & EMERGING FRONTIERS
- 2.5 GLOBAL PREIMPLANTATION GENETIC TESTING MARKET SNAPSHOT
3 PREMIUM INSIGHTS
- 3.1 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE AND COUNTRY
- 3.2 PREIMPLANTATION GENETIC TESTING MARKET: GEOGRAPHIC GROWTH OPPORTUNITIES
- 3.3 PREIMPLANTATION GENETIC TESTING MARKET SHARE, BY PRODUCT & SERVICE, 2025 VS. 2030 (%)
- 3.4 PREIMPLANTATION GENETIC TESTING MARKET SHARE, BY END USER, 2024 (%)
4 MARKET OVERVIEW
- 4.1 INTRODUCTION
- 4.2 MARKET DYNAMICS
- 4.2.1 DRIVERS
- 4.2.1.1 Decline in fertility rates
- 4.2.1.2 Expanding network of fertility clinics and IVF centers
- 4.2.1.3 Rising incidence of chromosomal abnormalities associated with advancing maternal age
- 4.2.2 RESTRAINTS
- 4.2.2.1 High procedural cost
- 4.2.2.2 Stringent regulatory frameworks governing IVF and genetic testing procedures
- 4.2.3 OPPORTUNITIES
- 4.2.3.1 Expanding acceptance of fertility treatments among single parents and same-sex couples
- 4.2.4 CHALLENGES
- 4.2.4.1 Socio-ethical concerns regarding embryo screening and selection
- 4.2.1 DRIVERS
- 4.3 UNMET NEEDS & WHITE SPACES
- 4.4 INTERCONNECTED MARKETS & CROSS-SECTOR OPPORTUNITIES
- 4.5 STRATEGIC MOVIES BY TIER-1/2/3 PLAYERS
5 INDUSTRY TRENDS
- 5.1 TRENDS/DISRUPTIONS IMPACTING CUSTOMER'S BUSINESS
- 5.2 MACROECONOMIC OUTLOOK
- 5.2.1 GDP TRENDS & FORECAST
- 5.2.2 R&D TRENDS IN GLOBAL HEALTHCARE INDUSTRY
- 5.2.3 R&D TRENDS IN GLOBAL PHARMA INDUSTRY
- 5.3 CASE STUDY ANALYSIS
- 5.3.1 PGT-M FOR RPGRIP1L VARIANT-ENABLED BY MINIGENE ASSAY
- 5.3.2 PERSISTENT FETAL MOSAICISM AFTER TRANSFER OF PGT-A MOSAIC EMBRYO
- 5.3.3 CRYPTIC REARRANGEMENTS RESOLVED BY OGM TO GUIDE PGT-SR
- 5.4 PRICING ANALYSIS
- 5.4.1 AVERAGE SELLING PRICE TREND OF PREIMPLANTATION GENETIC TESTING PRODUCTS, BY END USER, 2022-2024
- 5.4.2 AVERAGE SELLING PRICE TREND OF PREIMPLANTATION GENETIC TESTING PRODUCTS, BY KEY PLAYER, 2022-2024
- 5.4.3 AVERAGE SELLING PRICE TREND OF PREIMPLANTATION GENETIC TESTING PRODUCTS, BY PROCEDURE TYPE, 2022-2024
- 5.4.4 AVERAGE SELLING PRICE TREND OF PREIMPLANTATION GENETIC TESTING INSTRUMENTS AND CONSUMABLES, BY REGION, 2022-2024
- 5.5 TRADE ANALYSIS
- 5.5.1 IMPORT DATA FOR HS CODE 3822, 2020-2024
- 5.5.2 EXPORT DATA FOR HS CODE 3822, 2020-2024
- 5.5.3 IMPORT DATA FOR HS CODE 9018, 2020-2024
- 5.5.4 EXPORT DATA FOR HS CODE 9018, 2020-2024
- 5.6 VALUE CHAIN ANALYSIS
- 5.7 ECOSYSTEM ANALYSIS
- 5.7.1 ROLE IN ECOSYSTEM
- 5.8 PORTER'S FIVE FORCES ANALYSIS
- 5.8.1 INTENSITY OF COMPETITIVE RIVALRY
- 5.8.2 BARGAINING POWER OF SUPPLIERS
- 5.8.3 BARGAINING POWER OF BUYERS
- 5.8.4 THREAT OF SUBSTITUTES
- 5.8.5 THREAT OF NEW ENTRANTS
- 5.9 KEY CONFERENCES & EVENTS, 2025-2026
- 5.10 INVESTMENT & FUNDING SCENARIO
- 5.11 IMPACT OF 2025 US TARIFF ON PREIMPLANTATION GENETIC TESTING MARKET
- 5.11.1 KEY TARIFF RATES
- 5.11.2 PRICE IMPACT ANALYSIS
- 5.11.3 KEY IMPACTS ON VARIOUS REGIONS
- 5.11.3.1 US
- 5.11.3.2 Europe
- 5.11.3.3 Asia Pacific
- 5.11.4 END-USE INDUSTRY IMPACT
- 5.11.4.1 Fertility clinics
- 5.11.4.2 Hospitals
- 5.11.4.3 Diagnostic laboratories
6 TECHNOLOGICAL ADVANCEMENTS, AI-DRIVEN IMPACT, PATENTS, INNOVATIONS, AND FUTURE APPLICATIONS
- 6.1 TECHNOLOGY ANALYSIS
- 6.1.1 KEY TECHNOLOGIES
- 6.1.1.1 Laser-assisted TE biopsy
- 6.1.1.2 Whole genome amplification
- 6.1.2 COMPLEMENTARY TECHNOLOGIES
- 6.1.2.1 Micromanipulation
- 6.1.1 KEY TECHNOLOGIES
- 6.2 TECHNOLOGY ROADMAP
- 6.3 PATENT ANALYSIS
- 6.3.1 METHODOLOGY
- 6.3.2 NUMBER OF PATENTS FILED, BY DOCUMENT TYPE
- 6.3.3 LIST OF KEY PATENTS
- 6.4 FUTURE APPLICATIONS
- 6.5 IMPACT OF AI/GEN AI ON PREIMPLANTATION GENETIC TESTING MARKET
- 6.5.1 TOP USE CASES AND MARKET POTENTIAL
- 6.5.2 BEST PRACTICES IN AI-ENABLED EMBRYO SELECTION AND GENETIC SCREENING
- 6.5.3 CASE STUDIES OF AI IMPLEMENTATION IN PREIMPLANTATION GENETIC TESTING MARKET
- 6.5.4 INTERCONNECTED ADJACENT ECOSYSTEM AND IMPACT ON MARKET PLAYERS
- 6.5.5 CLIENTS' READINESS TO ADOPT GENERATIVE AI IN PREIMPLANTATION GENETIC TESTING MARKET
7 SUSTAINABILITY & REGULATORY LANDSCAPE
- 7.1 REGIONAL REGULATIONS & COMPLIANCE
- 7.1.1 REGULATORY BODIES, GOVERNMENT AGENCIES & OTHER ORGANIZATIONS
- 7.1.2 INDUSTRY STANDARDS
- 7.1.2.1 North America
- 7.1.2.1.1 US
- 7.1.2.1.2 Canada
- 7.1.2.2 Europe
- 7.1.2.3 Asia Pacific
- 7.1.2.3.1 Japan
- 7.1.2.3.2 China
- 7.1.2.3.3 India
- 7.1.2.4 Latin America
- 7.1.2.4.1 Brazil
- 7.1.2.1 North America
- 7.2 SUSTAINABILITY IMPACT & REGULATORY POLICY INITIATIVES
- 7.3 CERTIFICATIONS, LABELING, AND ECO-STANDARDS
8 CUSTOMER LANDSCAPE & BUYER BEHAVIOUR
- 8.1 DECISION-MAKING PROCESS
- 8.2 BUYER STAKEHOLDERS & BUYING EVALUATION CRITERIA
- 8.2.1 KEY STAKEHOLDERS IN BUYING PROCESS
- 8.2.2 KEY BUYING CRITERIA
- 8.3 ADOPTION BARRIERS & INTERNAL CHALLENGES
- 8.4 UNMET NEEDS FROM VARIOUS END-USE INDUSTRIES
9 PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE
- 9.1 INTRODUCTION
- 9.2 REAGENTS & CONSUMABLES
- 9.2.1 EXPANSION OF ADVANCED GENOMIC TECHNOLOGIES TO PROMOTE MARKET GROWTH
- 9.3 INSTRUMENTS
- 9.3.1 ONGOING TECHNOLOGICAL ADVANCEMENTS AND INCREASING FERTILITY CLINICS TO PROPEL MARKET GROWTH
- 9.4 SOFTWARE & SERVICES
- 9.4.1 RISING AWARENESS ABOUT DATA ANALYSIS TO DRIVE MARKET
10 PREIMPLANTATION GENETIC TESTING MARKET, BY PROCEDURE TYPE
- 10.1 INTRODUCTION
- 10.2 PREIMPLANTATION GENETIC SCREENING
- 10.2.1 INCREASING MATERNAL AGE TO AID MARKET GROWTH
- 10.3 PREIMPLANTATION GENETIC DIAGNOSIS
- 10.3.1 RISING AWARENESS ABOUT CHROMOSOMAL ABNORMALITIES IN FETUSES TO SUPPORT MARKET GROWTH
11 PREIMPLANTATION GENETIC TESTING MARKET, BY TECHNOLOGY
- 11.1 INTRODUCTION
- 11.2 NEXT-GENERATION SEQUENCING
- 11.2.1 IMPROVED TECHNOLOGY FOR DETECTING STRUCTURAL ABNORMALITIES TO FUEL MARKET GROWTH
- 11.3 POLYMERASE CHAIN REACTION
- 11.3.1 INCREASED USAGE IN CLINICAL AND RESEARCH APPLICATIONS AND HIGH PREVALENCE OF GENETIC DISEASES TO SUPPORT MARKET
- 11.4 FLUORESCENCE IN SITU HYBRIDIZATION
- 11.4.1 ADVANCES IN FLUORESCENCE MICROSCOPY AND DIGITAL IMAGING TO AUGMENT MARKET GROWTH
- 11.5 COMPARATIVE GENOMIC HYBRIDIZATION
- 11.5.1 LOW COST, LESS LABOR REQUIREMENT, AND ONGOING TECHNOLOGICAL ADVANCEMENTS TO DRIVE MARKET
- 11.6 SINGLE-NUCLEOTIDE POLYMORPHISM
- 11.6.1 HIGH-RESOLUTION ACCURACY AND VALIDATED CLINICAL PERFORMANCE TO BOOST MARKET GROWTH
12 PREIMPLANTATION GENETIC TESTING MARKET, BY APPLICATION
- 12.1 INTRODUCTION
- 12.2 ANEUPLOIDY
- 12.2.1 HIGH ANEUPLOIDY INCIDENCE AND RAPID INNOVATION TO ACCELERATE MARKET GROWTH
- 12.3 STRUCTURAL CHROMOSOMAL ABNORMALITIES
- 12.3.1 TRANSLOCATIONS
- 12.3.1.1 Rising incidence of translocation chromosomal abnormalities during IVF treatments to drive segment
- 12.3.2 DELETIONS
- 12.3.2.1 Advancements in genetic testing to boost segment growth
- 12.3.3 DUPLICATIONS
- 12.3.3.1 Rising cases of duplication of chromosomal abnormalities and increasing maternal age to drive segment
- 12.3.4 INVERSIONS
- 12.3.4.1 Risk of unexplained male-factor infertility and multiple miscarriages to limit segment growth
- 12.3.1 TRANSLOCATIONS
- 12.4 SINGLE GENE DISORDERS
- 12.4.1 INCREASING AWARENESS ABOUT GENETIC TESTING TO PROPEL MARKET GROWTH
- 12.5 X-LINKED DISORDERS
- 12.5.1 MEDICAL ADVANCEMENTS AND INCREASED RESEARCH ON GENETIC DISORDERS TO AUGMENT MARKET GROWTH
- 12.6 HLA TYPING
- 12.6.1 RISING NUMBER OF COUPLES WITH CHILDREN AFFECTED BY HEMATOLOGICAL DISEASES TO DRIVE MARKET
- 12.7 GENDER IDENTIFICATION
- 12.7.1 INCREASED FOCUS ON SEX DISCRIMINATION TO LIMIT MARKET GROWTH
13 PREIMPLANTATION GENETIC TESTING MARKET, BY TYPE OF CYCLE
- 13.1 INTRODUCTION
- 13.2 FRESH NON-DONOR
- 13.2.1 HIGH RATES OF SUCCESSFUL PREGNANCY AMONG YOUNG WOMEN TO DRIVE MARKET GROWTH
- 13.3 FROZEN NON-DONOR
- 13.3.1 LONG-TERM STORAGE OF FROZEN EGGS AND EASY SCHEDULING TO PROPEL MARKET GROWTH
- 13.4 FRESH DONOR
- 13.4.1 HIGH-QUALITY FRESH DONOR OOCYTES AND EXPANDING PGT-DRIVEN RISK REDUCTION TO PROMOTE MARKET GROWTH
- 13.5 FROZEN DONOR
- 13.5.1 RISING USE OF ADVANCED AND NON-INVASIVE PGT TECHNOLOGIES TO AID MARKET GROWTH
14 PREIMPLANTATION GENETIC TESTING MARKET, BY END USER
- 14.1 INTRODUCTION
- 14.2 FERTILITY CLINICS
- 14.2.1 HIGH SUCCESS RATE OF FERTILITY TREATMENT TO DRIVE MARKET
- 14.3 DIAGNOSTIC LABORATORIES
- 14.3.1 IMPROVED CLINICAL EFFICACY AND INCREASED RESEARCH FUNDING TO FUEL MARKET
- 14.4 HOSPITALS
- 14.4.1 RISING NUMBER OF HOSPITALS AND INCREASING HEALTHCARE AWARENESS TO DRIVE GROWTH
- 14.5 OTHER END USERS
15 PREIMPLANTATION GENETIC TESTING MARKET, BY REGION
- 15.1 INTRODUCTION
- 15.2 NORTH AMERICA
- 15.2.1 US
- 15.2.1.1 US to dominate North American preimplantation genetic testing market during forecast period
- 15.2.2 CANADA
- 15.2.2.1 Whole-genome embryo testing and public-private R&D investment to propel market growth
- 15.2.1 US
- 15.3 EUROPE
- 15.3.1 GERMANY
- 15.3.1.1 Global clinical adoption of precision medicines to boost market growth
- 15.3.2 UK
- 15.3.2.1 Demographic pressures and evidence-based clinical innovation to support market growth
- 15.3.3 FRANCE
- 15.3.3.1 Ethical regulation and adoption of patient-centered precision medicines to fuel market growth
- 15.3.4 ITALY
- 15.3.4.1 Regulatory reforms and clinical-genetic partnerships to boost market growth
- 15.3.5 SPAIN
- 15.3.5.1 Well-established network of research centers and universities to augment market growth
- 15.3.6 REST OF EUROPE
- 15.3.1 GERMANY
- 15.4 ASIA PACIFIC
- 15.4.1 CHINA
- 15.4.1.1 Advancing next-generation genomic infrastructure and cross-border fertility capacity to propel market growth
- 15.4.2 JAPAN
- 15.4.2.1 Reinforcing global PGT standards and rigorous clinical governance to favor market growth
- 15.4.3 INDIA
- 15.4.3.1 Expanding genetic-diagnostic access to drive market growth
- 15.4.4 AUSTRALIA
- 15.4.4.1 Standardized regulation and expanding clinical adoption to fuel market growth
- 15.4.5 SOUTH KOREA
- 15.4.5.1 Clinically validated PGT-A integration and improved IVF outcomes to support market growth
- 15.4.6 REST OF ASIA PACIFIC
- 15.4.1 CHINA
- 15.5 LATIN AMERICA
- 15.5.1 BRAZIL
- 15.5.1.1 Global genomic innovation and regulatory modernization to boost market growth
- 15.5.2 MEXICO
- 15.5.2.1 Adoption of advanced NGS-based PGT services to support growth
- 15.5.3 REST OF LATIN AMERICA
- 15.5.1 BRAZIL
- 15.6 MIDDLE EAST
- 15.6.1 GCC COUNTRIES
- 15.6.1.1 Kingdom of Saudi Arabia
- 15.6.1.1.1 Rising global emphasis on preventive genomic medicine and advanced embryo-screening technologies to drive market
- 15.6.1.2 UAE
- 15.6.1.2.1 Rising global demand for safer and technologically advanced embryo screening to aid market growth
- 15.6.1.3 Rest of GCC Countries
- 15.6.1.1 Kingdom of Saudi Arabia
- 15.6.2 REST OF MIDDLE EAST
- 15.6.1 GCC COUNTRIES
- 15.7 AFRICA
- 15.7.1 REGIONAL ART REGISTRY EXPANSION AND RISING IVF INFRASTRUCTURE TO PROPEL MARKET GROWTH
16 COMPETITIVE LANDSCAPE
- 16.1 INTRODUCTION
- 16.2 OVERVIEW OF STRATEGIES ADOPTED BY KEY PLAYERS IN PREIMPLANTATION GENETIC TESTING MARKET
- 16.3 REVENUE ANALYSIS, 2020-2024
- 16.4 MARKET SHARE ANALYSIS, 2024
- 16.5 COMPANY VALUATION & FINANCIAL METRICS
- 16.5.1 FINANCIAL METRICS
- 16.5.2 COMPANY VALUATION
- 16.6 BRAND/PRODUCT COMPARISON
- 16.6.1 ILLUMINA, INC.
- 16.6.2 THERMO FISHER SCIENTIFIC INC.
- 16.6.3 AGILENT TECHNOLOGIES, INC.
- 16.7 COMPANY EVALUATION MATRIX: KEY PLAYERS, 2024
- 16.7.1 STARS
- 16.7.2 EMERGING LEADERS
- 16.7.3 PERVASIVE PLAYERS
- 16.7.4 PARTICIPANTS
- 16.7.5 COMPANY FOOTPRINT: KEY PLAYERS, 2024
- 16.7.5.1 Company footprint
- 16.7.5.2 Region footprint
- 16.7.5.3 Product & service footprint
- 16.7.5.4 Technology footprint
- 16.7.5.5 Application footprint
- 16.8 COMPANY EVALUATION MATRIX: STARTUPS/SMES, 2024
- 16.8.1 PROGRESSIVE COMPANIES
- 16.8.2 RESPONSIVE COMPANIES
- 16.8.3 DYNAMIC COMPANIES
- 16.8.4 STARTING BLOCKS
- 16.8.5 COMPETITIVE BENCHMARKING
- 16.8.5.1 Detailed list of key startups/SMEs
- 16.8.5.2 Competitive benchmarking of key startups/SMEs
- 16.9 COMPETITIVE SCENARIO
- 16.9.1 PRODUCT LAUNCHES
- 16.9.2 DEALS
- 16.9.3 EXPANSIONS
17 COMPANY PROFILES
- 17.1 KEY PLAYERS
- 17.1.1 ILLUMINA, INC.
- 17.1.1.1 Business overview
- 17.1.1.2 Products/Services/Solutions offered
- 17.1.1.3 Recent developments
- 17.1.1.3.1 Deals
- 17.1.1.4 MnM view
- 17.1.1.4.1 Key strengths
- 17.1.1.4.2 Strategic choices
- 17.1.1.4.3 Weaknesses & competitive threats
- 17.1.2 THERMO FISHER SCIENTIFIC INC.
- 17.1.2.1 Business overview
- 17.1.2.2 Products/Services/Solutions offered
- 17.1.2.3 Recent developments
- 17.1.2.3.1 Product launches
- 17.1.2.4 MnM view
- 17.1.2.4.1 Key strengths
- 17.1.2.4.2 Strategic choices
- 17.1.2.4.3 Weaknesses & competitive threats
- 17.1.3 AGILENT TECHNOLOGIES, INC.
- 17.1.3.1 Business overview
- 17.1.3.2 Products/Services/Solutions offered
- 17.1.3.3 MnM view
- 17.1.3.3.1 Key strengths
- 17.1.3.3.2 Strategic choices
- 17.1.3.3.3 Weaknesses & competitive threats
- 17.1.4 REVVITY
- 17.1.4.1 Business overview
- 17.1.4.2 Products/Services/Solutions offered
- 17.1.4.3 MnM view
- 17.1.4.3.1 Key strengths
- 17.1.4.3.2 Strategic choices
- 17.1.4.3.3 Weaknesses & competitive threats
- 17.1.5 COOPERCOMPANIES
- 17.1.5.1 Business overview
- 17.1.5.2 Products/Services/Solutions offered
- 17.1.5.3 MnM view
- 17.1.5.3.1 Key strengths
- 17.1.5.3.2 Strategic choices
- 17.1.5.3.3 Weaknesses & competitive threats
- 17.1.6 ABBOTT
- 17.1.6.1 Business overview
- 17.1.6.2 Products/Services/Solutions offered
- 17.1.7 TAKARA BIO INC.
- 17.1.7.1 Business overview
- 17.1.7.2 Products/Services/Solutions offered
- 17.1.8 QIAGEN
- 17.1.8.1 Business overview
- 17.1.8.2 Products/Services/Solutions offered
- 17.1.9 VITROLIFE
- 17.1.9.1 Business overview
- 17.1.9.2 Products/Services/Solutions offered
- 17.1.10 OXFORD NANOPORE TECHNOLOGIES PLC
- 17.1.10.1 Business overview
- 17.1.10.2 Products/Services/Solutions offered
- 17.1.10.3 Recent developments
- 17.1.10.3.1 Expansions
- 17.1.1 ILLUMINA, INC.
- 17.2 OTHER PLAYERS
- 17.2.1 OXFORD GENE TECHNOLOGY IP LIMITED
- 17.2.2 YIKON GENOMICS
- 17.2.3 SHIVA SCIENTIFIC
- 17.2.4 NANJING SUPERYEARS GENE TECHNOLOGY CO., LTD.
- 17.2.5 MEDICOVER GENETICS
- 17.2.6 MEDGENOME
- 17.2.7 FULGENT GENETICS
- 17.2.8 INVICTA SP. Z O.O.
- 17.2.9 GENEA PTY LIMITED
- 17.2.10 SCIGENE CORPORATION
- 17.2.11 BIOARRAY S.L.
- 17.2.12 UNIMED BIOTECH (SHANGHAI) CO., LTD.
- 17.2.13 GENEMIND BIOSCIENCES CO., LTD.
- 17.2.14 BERRY GENOMICS
- 17.2.15 BANGKOK GENOMICS INNOVATION
18 APPENDIX
- 18.1 DISCUSSION GUIDE
- 18.2 KNOWLEDGESTORE: MARKETSANDMARKETS' SUBSCRIPTION PORTAL
- 18.3 CUSTOMIZATION OPTIONS
- 18.4 RELATED REPORTS
- 18.5 AUTHOR DETAILS
List of Tables
- TABLE 1 PREIMPLANTATION GENETIC TESTING MARKET: INCLUSIONS & EXCLUSIONS
- TABLE 2 PREIMPLANTATION GENETIC TESTING MARKET: IMPACT ANALYSIS OF MARKET DYNAMICS
- TABLE 3 AVERAGE SELLING PRICE TREND OF PREIMPLANTATION GENETIC TESTING PRODUCTS, BY END USER, 2022-2024 (USD)
- TABLE 4 AVERAGE SELLING PRICE TREND OF PREIMPLANTATION GENETIC TESTING PRODUCTS, BY KEY PLAYER, 2022-2024 (USD)
- TABLE 5 AVERAGE SELLING PRICE TREND OF PREIMPLANTATION GENETIC TESTING PRODUCTS, BY PROCEDURE TYPE, 2022-2024 (USD)
- TABLE 6 AVERAGE SELLING PRICE TREND OF PREIMPLANTATION GENETIC TESTING INSTRUMENTS, BY REGION, 2022-2024 (USD MILLION)
- TABLE 7 AVERAGE SELLING PRICE MEAN TREND OF PREIMPLANTATION GENETIC TESTING CONSUMABLES, BY REGION, 2022-2024 (USD)
- TABLE 8 IMPORT DATA FOR HS CODE 3822, BY COUNTRY, 2020-2024 (USD THOUSAND)
- TABLE 9 EXPORT DATA FOR HS CODE 3822, BY COUNTRY, 2020-2024 (USD THOUSAND)
- TABLE 10 IMPORT DATA FOR HS CODE 9018, BY COUNTRY, 2020-2024 (USD THOUSAND)
- TABLE 11 EXPORT DATA FOR HS CODE 9018, BY COUNTRY, 2020-2024 (USD THOUSAND)
- TABLE 12 PREIMPLANTATION GENETIC TESTING MARKET: ROLE IN ECOSYSTEM
- TABLE 13 PREIMPLANTATION GENETIC TESTING MARKET: PORTER'S FIVE FORCES
- TABLE 14 KEY CONFERENCES & EVENTS IN PREIMPLANTATION GENETIC TESTING MARKET: JANUARY 2025-DECEMBER 2026
- TABLE 15 US-ADJUSTED RECIPROCAL TARIFF RATES
- TABLE 16 NUMBER OF EXPORTS AND IMPORTS, BY REGION, 2024-2025 (USD MILLION)
- TABLE 17 KEY PRODUCT-RELATED TARIFF: HS CODES FOR PRODUCTS RELEVANT TO PREIMPLANTATION GENETIC TESTING
- TABLE 18 CRITICAL COMPONENTS LIKELY EXPOSED TO TARIFF CHANGES
- TABLE 19 NUMBER OF PATENTS FILED, BY DOCUMENT TYPE, 2014-2024
- TABLE 20 LIST OF KEY PATENTS IN PREIMPLANTATION GENETIC TESTING MARKET, 2023-2025
- TABLE 21 KEY PLAYERS IMPLEMENTING AI/GEN AI IN PREIMPLANTATION GENETIC TESTING MARKET
- TABLE 22 NORTH AMERICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 23 EUROPE: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 24 ASIA PACIFIC: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 25 REST OF THE WORLD: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 26 US: CLASSIFICATION OF IN VITRO DIAGNOSTIC DEVICES
- TABLE 27 EUROPE: CLASSIFICATION OF IN VITRO DIAGNOSTIC DEVICES
- TABLE 28 JAPAN: CLASSIFICATION OF IN VITRO DIAGNOSTIC REAGENTS
- TABLE 29 JAPAN: TIME, COST, AND COMPLEXITY OF REGISTRATION PROCESS
- TABLE 30 CHINA: TIME, COST, AND COMPLEXITY OF REGISTRATION PROCESS
- TABLE 31 VENDOR CERTIFICATIONS: PREIMPLANTATION GENETIC TESTING MARKET
- TABLE 32 INFLUENCE OF KEY STAKEHOLDERS ON BUYING PROCESS, BY PRODUCT & SERVICE
- TABLE 33 KEY BUYING CRITERIA FOR MAJOR END USERS
- TABLE 34 PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 35 PREIMPLANTATION GENETIC TESTING REAGENTS & CONSUMABLES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 36 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING REAGENTS & CONSUMABLES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 37 EUROPE: PREIMPLANTATION GENETIC TESTING REAGENTS & CONSUMABLES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 38 ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING REAGENTS & CONSUMABLES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 39 LATIN AMERICA: PREIMPLANTATION GENETIC TESTING REAGENTS & CONSUMABLES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 40 MIDDLE EAST: PREIMPLANTATION GENETIC TESTING REAGENTS & CONSUMABLES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 41 GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING REAGENTS & CONSUMABLES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 42 PREIMPLANTATION GENETIC TESTING INSTRUMENTS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 43 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING INSTRUMENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 44 EUROPE: PREIMPLANTATION GENETIC TESTING INSTRUMENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 45 ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING INSTRUMENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 46 LATIN AMERICA: PREIMPLANTATION GENETIC TESTING INSTRUMENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 47 MIDDLE EAST: PREIMPLANTATION GENETIC TESTING INSTRUMENTS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 48 GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING INSTRUMENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 49 PREIMPLANTATION GENETIC TESTING SOFTWARE & SERVICES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 50 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING SOFTWARE & SERVICES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 51 EUROPE: PREIMPLANTATION GENETIC TESTING SOFTWARE & SERVICES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 52 ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING SOFTWARE & SERVICES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 53 LATIN AMERICA: PREIMPLANTATION GENETIC TESTING SOFTWARE & SERVICES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 54 MIDDLE EAST: PREIMPLANTATION GENETIC TESTING SOFTWARE & SERVICES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 55 GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING SOFTWARE & SERVICES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 56 PREIMPLANTATION GENETIC TESTING MARKET, BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
- TABLE 57 PREIMPLANTATION GENETIC SCREENING MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 58 NORTH AMERICA: PREIMPLANTATION GENETIC SCREENING MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 59 EUROPE: PREIMPLANTATION GENETIC SCREENING MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 60 ASIA PACIFIC: PREIMPLANTATION GENETIC SCREENING MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 61 LATIN AMERICA: PREIMPLANTATION GENETIC SCREENING MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 62 MIDDLE EAST: PREIMPLANTATION GENETIC SCREENING MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 63 GCC COUNTRIES: PREIMPLANTATION GENETIC SCREENING MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 64 PREIMPLANTATION GENETIC DIAGNOSIS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 65 NORTH AMERICA: PREIMPLANTATION GENETIC DIAGNOSIS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 66 EUROPE: PREIMPLANTATION GENETIC DIAGNOSIS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 67 ASIA PACIFIC: PREIMPLANTATION GENETIC DIAGNOSIS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 68 LATIN AMERICA: PREIMPLANTATION GENETIC DIAGNOSIS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 69 MIDDLE EAST: PREIMPLANTATION GENETIC DIAGNOSIS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 70 GCC COUNTRIES: PREIMPLANTATION GENETIC DIAGNOSIS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 71 PREIMPLANTATION GENETIC TESTING MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 72 PREIMPLANTATION GENETIC TESTING MARKET FOR NEXT-GENERATION SEQUENCING, BY REGION, 2023-2030 (USD MILLION)
- TABLE 73 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR NEXT-GENERATION SEQUENCING, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 74 EUROPE: PREIMPLANTATION GENETIC TESTING MARKET FOR NEXT-GENERATION SEQUENCING, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 75 ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET FOR NEXT-GENERATION SEQUENCING, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 76 LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR NEXT-GENERATION SEQUENCING, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 77 MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET FOR NEXT-GENERATION SEQUENCING, BY REGION, 2023-2030 (USD MILLION)
- TABLE 78 GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET FOR NEXT-GENERATION SEQUENCING, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 79 PREIMPLANTATION GENETIC TESTING MARKET FOR POLYMERASE CHAIN REACTION, BY REGION, 2023-2030 (USD MILLION)
- TABLE 80 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR POLYMERASE CHAIN REACTION, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 81 EUROPE: PREIMPLANTATION GENETIC TESTING MARKET FOR POLYMERASE CHAIN REACTION, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 82 ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET FOR POLYMERASE CHAIN REACTION, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 83 LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR POLYMERASE CHAIN REACTION, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 84 MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET FOR POLYMERASE CHAIN REACTION, BY REGION, 2023-2030 (USD MILLION)
- TABLE 85 GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET FOR POLYMERASE CHAIN REACTION, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 86 PREIMPLANTATION GENETIC TESTING MARKET FOR FLUORESCENCE IN SITU HYBRIDIZATION, BY REGION, 2023-2030 (USD MILLION)
- TABLE 87 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR FLUORESCENCE IN SITU HYBRIDIZATION, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 88 EUROPE: PREIMPLANTATION GENETIC TESTING MARKET FOR FLUORESCENCE IN SITU HYBRIDIZATION, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 89 ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET FOR FLUORESCENCE IN SITU HYBRIDIZATION, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 90 LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR FLUORESCENCE IN SITU HYBRIDIZATION, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 91 MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET FOR FLUORESCENCE IN SITU HYBRIDIZATION, BY REGION, 2023-2030 (USD MILLION)
- TABLE 92 GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET FOR FLUORESCENCE IN SITU HYBRIDIZATION, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 93 PREIMPLANTATION GENETIC TESTING MARKET FOR COMPARATIVE GENOMIC HYBRIDIZATION, BY REGION, 2023-2030 (USD MILLION)
- TABLE 94 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR COMPARATIVE GENOMIC HYBRIDIZATION, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 95 EUROPE: PREIMPLANTATION GENETIC TESTING MARKET FOR COMPARATIVE GENOMIC HYBRIDIZATION, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 96 ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET FOR COMPARATIVE GENOMIC HYBRIDIZATION, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 97 LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR COMPARATIVE GENOMIC HYBRIDIZATION, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 98 MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET FOR COMPARATIVE GENOMIC HYBRIDIZATION, BY REGION, 2023-2030 (USD MILLION)
- TABLE 99 GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET FOR COMPARATIVE GENOMIC HYBRIDIZATION, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 100 PREIMPLANTATION GENETIC TESTING MARKET FOR SINGLE-NUCLEOTIDE POLYMORPHISM, BY REGION, 2023-2030 (USD MILLION)
- TABLE 101 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR SINGLE-NUCLEOTIDE POLYMORPHISM, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 102 EUROPE: PREIMPLANTATION GENETIC TESTING MARKET FOR SINGLE-NUCLEOTIDE POLYMORPHISM, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 103 ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET FOR SINGLE-NUCLEOTIDE POLYMORPHISM, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 104 LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR SINGLE-NUCLEOTIDE POLYMORPHISM, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 105 MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET FOR SINGLE-NUCLEOTIDE POLYMORPHISM, BY REGION, 2023-2030 (USD MILLION)
- TABLE 106 GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET FOR SINGLE-NUCLEOTIDE POLYMORPHISM, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 107 PREIMPLANTATION GENETIC TESTING MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 108 PREIMPLANTATION GENETIC TESTING MARKET FOR ANEUPLOIDY, BY REGION, 2023-2030 (USD MILLION)
- TABLE 109 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR ANEUPLOIDY, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 110 EUROPE: PREIMPLANTATION GENETIC TESTING MARKET FOR ANEUPLOIDY, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 111 ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET FOR ANEUPLOIDY, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 112 LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR ANEUPLOIDY, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 113 MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET FOR ANEUPLOIDY, BY REGION, 2023-2030 (USD MILLION)
- TABLE 114 GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET FOR ANEUPLOIDY, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 115 PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 116 PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 117 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 118 EUROPE: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 119 ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 120 LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 121 MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 122 GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 123 STRUCTURAL CHROMOSOMAL ABNORMALITIES MARKET FOR TRANSLOCATIONS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 124 NORTH AMERICA: STRUCTURAL CHROMOSOMAL ABNORMALITIES MARKET FOR TRANSLOCATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 125 EUROPE: STRUCTURAL CHROMOSOMAL ABNORMALITIES MARKET FOR TRANSLOCATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 126 ASIA PACIFIC: STRUCTURAL CHROMOSOMAL ABNORMALITIES MARKET FOR TRANSLOCATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 127 LATIN AMERICA: STRUCTURAL CHROMOSOMAL ABNORMALITIES MARKET FOR TRANSLOCATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 128 MIDDLE EAST: STRUCTURAL CHROMOSOMAL ABNORMALITIES MARKET FOR TRANSLOCATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 129 GCC COUNTRIES: STRUCTURAL CHROMOSOMAL ABNORMALITIES MARKET FOR TRANSLOCATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 130 STRUCTURAL CHROMOSOMAL ABNORMALITIES MARKET FOR DELETIONS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 131 NORTH AMERICA: STRUCTURAL CHROMOSOMAL ABNORMALITIES MARKET FOR DELETIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 132 EUROPE: STRUCTURAL CHROMOSOMAL ABNORMALITIES MARKET FOR DELETIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 133 ASIA PACIFIC: STRUCTURAL CHROMOSOMAL ABNORMALITIES MARKET FOR DELETIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 134 LATIN AMERICA: STRUCTURAL CHROMOSOMAL ABNORMALITIES MARKET FOR DELETIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 135 MIDDLE EAST: STRUCTURAL CHROMOSOMAL ABNORMALITIES MARKET FOR DELETIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 136 GCC COUNTRIES: STRUCTURAL CHROMOSOMAL ABNORMALITIES MARKET FOR DELETIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 137 STRUCTURAL CHROMOSOMAL ABNORMALITIES MARKET FOR DUPLICATIONS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 138 NORTH AMERICA: STRUCTURAL CHROMOSOMAL ABNORMALITIES MARKET FOR DUPLICATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 139 EUROPE: STRUCTURAL CHROMOSOMAL ABNORMALITIES MARKET FOR DUPLICATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 140 ASIA PACIFIC: STRUCTURAL CHROMOSOMAL ABNORMALITIES MARKET FOR DUPLICATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 141 LATIN AMERICA: STRUCTURAL CHROMOSOMAL ABNORMALITIES MARKET FOR DUPLICATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 142 MIDDLE EAST: STRUCTURAL CHROMOSOMAL ABNORMALITIES MARKET FOR DUPLICATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 143 GCC COUNTRIES: STRUCTURAL CHROMOSOMAL ABNORMALITIES MARKET FOR DUPLICATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 144 STRUCTURAL CHROMOSOMAL ABNORMALITIES MARKET FOR INVERSIONS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 145 NORTH AMERICA: STRUCTURAL CHROMOSOMAL ABNORMALITIES MARKET FOR INVERSIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 146 EUROPE: STRUCTURAL CHROMOSOMAL ABNORMALITIES MARKET FOR INVERSIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 147 ASIA PACIFIC: STRUCTURAL CHROMOSOMAL ABNORMALITIES MARKET FOR INVERSIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 148 LATIN AMERICA: STRUCTURAL CHROMOSOMAL ABNORMALITIES MARKET INVERSIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 149 MIDDLE EAST: STRUCTURAL CHROMOSOMAL ABNORMALITIES MARKET FOR INVERSIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 150 GCC COUNTRIES: STRUCTURAL CHROMOSOMAL ABNORMALITIES MARKET FOR INVERSIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 151 PREIMPLANTATION GENETIC TESTING MARKET FOR SINGLE GENE DISORDERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 152 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR SINGLE GENE DISORDERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 153 EUROPE: PREIMPLANTATION GENETIC TESTING MARKET FOR SINGLE GENE DISORDERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 154 ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET FOR SINGLE GENE DISORDERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 155 LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR SINGLE GENE DISORDERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 156 MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET FOR SINGLE GENE DISORDERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 157 GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET FOR SINGLE GENE DISORDERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 158 PREIMPLANTATION GENETIC TESTING MARKET FOR X-LINKED DISORDERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 159 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR X-LINKED DISORDERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 160 EUROPE: PREIMPLANTATION GENETIC TESTING MARKET FOR X-LINKED DISORDERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 161 ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET FOR X-LINKED DISORDERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 162 LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR X-LINKED DISORDERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 163 MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET FOR X-LINKED DISORDERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 164 GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET FOR X-LINKED DISORDERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 165 PREIMPLANTATION GENETIC TESTING MARKET FOR HLA TYPING, BY REGION, 2023-2030 (USD MILLION)
- TABLE 166 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR HLA TYPING, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 167 EUROPE: PREIMPLANTATION GENETIC TESTING MARKET FOR HLA TYPING, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 168 ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET FOR HLA TYPING, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 169 LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR HLA TYPING, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 170 MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET FOR HLA TYPING, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 171 GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET FOR HLA TYPING, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 172 PREIMPLANTATION GENETIC TESTING MARKET FOR GENDER IDENTIFICATION, BY REGION, 2023-2030 (USD MILLION)
- TABLE 173 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR GENDER IDENTIFICATION, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 174 EUROPE: PREIMPLANTATION GENETIC TESTING MARKET FOR GENDER IDENTIFICATION, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 175 ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET FOR GENDER IDENTIFICATION, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 176 LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR GENDER IDENTIFICATION, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 177 MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET FOR GENDER IDENTIFICATION, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 178 GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET FOR GENDER IDENTIFICATION, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 179 PREIMPLANTATION GENETIC TESTING MARKET, BY TYPE OF CYCLE, 2023-2030 (USD MILLION)
- TABLE 180 PREIMPLANTATION GENETIC TESTING MARKET FOR FRESH NON-DONOR, BY REGION, 2023-2030 (USD MILLION)
- TABLE 181 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR FRESH NON-DONOR, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 182 EUROPE: PREIMPLANTATION GENETIC TESTING MARKET FOR FRESH NON-DONOR, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 183 ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET FOR FRESH NON-DONOR, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 184 LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR FRESH NON-DONOR, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 185 MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET FOR FRESH NON-DONOR, BY REGION, 2023-2030 (USD MILLION)
- TABLE 186 GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET FOR FRESH NON-DONOR, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 187 PREIMPLANTATION GENETIC TESTING MARKET FOR FROZEN NON-DONOR, BY REGION, 2023-2030 (USD MILLION)
- TABLE 188 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR FROZEN NON-DONOR, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 189 EUROPE: PREIMPLANTATION GENETIC TESTING MARKET FOR FROZEN NON-DONOR, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 190 ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET FOR FROZEN NON-DONOR, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 191 LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR FROZEN NON-DONOR, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 192 MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET FOR FROZEN NON-DONOR, BY REGION, 2023-2030 (USD MILLION)
- TABLE 193 GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET FOR FROZEN NON-DONOR, SBY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 194 PREIMPLANTATION GENETIC TESTING MARKET FOR FRESH DONOR, BY REGION, 2023-2030 (USD MILLION)
- TABLE 195 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR FRESH DONOR, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 196 EUROPE: PREIMPLANTATION GENETIC TESTING MARKET FOR FRESH DONOR, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 197 ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET FOR FRESH DONOR, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 198 LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR FRESH DONOR, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 199 MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET FOR FRESH DONOR, BY REGION, 2023-2030 (USD MILLION)
- TABLE 200 GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET FOR FRESH DONOR, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 201 PREIMPLANTATION GENETIC TESTING MARKET FOR FROZEN DONOR, BY REGION, 2023-2030 (USD MILLION)
- TABLE 202 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR FROZEN DONOR, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 203 EUROPE: PREIMPLANTATION GENETIC TESTING MARKET FOR FROZEN DONOR, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 204 ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET FOR FROZEN DONOR, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 205 LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR FROZEN DONOR, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 206 MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET FOR FROZEN DONOR, BY REGION, 2023-2030 (USD MILLION)
- TABLE 207 GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET FOR FROZEN DONOR, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 208 PREIMPLANTATION GENETIC TESTING MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 209 PREIMPLANTATION GENETIC TESTING MARKET FOR FERTILITY CLINICS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 210 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR FERTILITY CLINICS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 211 EUROPE: PREIMPLANTATION GENETIC TESTING MARKET FOR FERTILITY CLINICS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 212 ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET FOR FERTILITY CLINICS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 213 LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR FERTILITY CLINICS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 214 MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET FOR FERTILITY CLINICS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 215 GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET FOR FERTILITY CLINICS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 216 PREIMPLANTATION GENETIC TESTING MARKET FOR DIAGNOSTIC LABORATORIES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 217 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR DIAGNOSTIC LABORATORIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 218 EUROPE: PREIMPLANTATION GENETIC TESTING MARKET FOR DIAGNOSTIC LABORATORIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 219 ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET FOR DIAGNOSTIC LABORATORIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 220 LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR DIAGNOSTIC LABORATORIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 221 MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET FOR DIAGNOSTIC LABORATORIES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 222 GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET FOR DIAGNOSTIC LABORATORIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 223 PREIMPLANTATION GENETIC TESTING MARKET FOR HOSPITALS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 224 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR HOSPITALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 225 EUROPE: PREIMPLANTATION GENETIC TESTING MARKET FOR HOSPITALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 226 ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET FOR HOSPITALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 227 LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR HOSPITALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 228 MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET FOR HOSPITALS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 229 GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET FOR HOSPITALS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 230 PREIMPLANTATION GENETIC TESTING MARKET FOR OTHER END USERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 231 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR OTHER END USERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 232 EUROPE: PREIMPLANTATION GENETIC TESTING MARKET FOR OTHER END USERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 233 ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET FOR OTHER END USERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 234 LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR OTHER END USERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 235 MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET FOR OTHER END USERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 236 GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET FOR OTHER END USERS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 237 PREIMPLANTATION GENETIC TESTING MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 238 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 239 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 240 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING MARKET, BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
- TABLE 241 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 242 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 243 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 244 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING MARKET, BY TYPE OF CYCLE, 2023-2030 (USD MILLION)
- TABLE 245 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 246 US: PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 247 US: PREIMPLANTATION GENETIC TESTING MARKET, BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
- TABLE 248 US: PREIMPLANTATION GENETIC TESTING MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 249 US: PREIMPLANTATION GENETIC TESTING MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 250 US: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 251 US: PREIMPLANTATION GENETIC TESTING MARKET, BY TYPE OF CYCLE, 2023-2030 (USD MILLION)
- TABLE 252 US: PREIMPLANTATION GENETIC TESTING MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 253 CANADA: PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 254 CANADA: PREIMPLANTATION GENETIC TESTING MARKET, BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
- TABLE 255 CANADA: PREIMPLANTATION GENETIC TESTING MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 256 CANADA: PREIMPLANTATION GENETIC TESTING MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 257 CANADA: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 258 CANADA: PREIMPLANTATION GENETIC TESTING MARKET, BY TYPE OF CYCLE, 2023-2030 (USD MILLION)
- TABLE 259 CANADA: PREIMPLANTATION GENETIC TESTING MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 260 EUROPE: PREIMPLANTATION GENETIC TESTING MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 261 EUROPE: PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 262 EUROPE: PREIMPLANTATION GENETIC TESTING MARKET, BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
- TABLE 263 EUROPE: PREIMPLANTATION GENETIC TESTING MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 264 EUROPE: PREIMPLANTATION GENETIC TESTING MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 265 EUROPE: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 266 EUROPE: PREIMPLANTATION GENETIC TESTING MARKET, BY TYPE OF CYCLE, 2023-2030 (USD MILLION)
- TABLE 267 EUROPE: PREIMPLANTATION GENETIC TESTING MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 268 GERMANY: PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 269 GERMANY: PREIMPLANTATION GENETIC TESTING MARKET, BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
- TABLE 270 GERMANY: PREIMPLANTATION GENETIC TESTING MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 271 GERMANY: PREIMPLANTATION GENETIC TESTING MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 272 GERMANY: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 273 GERMANY: PREIMPLANTATION GENETIC TESTING MARKET, BY TYPE OF CYCLE, 2023-2030 (USD MILLION)
- TABLE 274 GERMANY: PREIMPLANTATION GENETIC TESTING MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 275 UK: PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 276 UK: PREIMPLANTATION GENETIC TESTING MARKET, BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
- TABLE 277 UK: PREIMPLANTATION GENETIC TESTING MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 278 UK: PREIMPLANTATION GENETIC TESTING MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 279 UK: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 280 UK: PREIMPLANTATION GENETIC TESTING MARKET, BY TYPE OF CYCLE, 2023-2030 (USD MILLION)
- TABLE 281 UK: PREIMPLANTATION GENETIC TESTING MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 282 FRANCE: PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 283 FRANCE: PREIMPLANTATION GENETIC TESTING MARKET, BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
- TABLE 284 FRANCE: PREIMPLANTATION GENETIC TESTING MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 285 FRANCE: PREIMPLANTATION GENETIC TESTING MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 286 FRANCE: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 287 FRANCE: PREIMPLANTATION GENETIC TESTING MARKET, BY TYPE OF CYCLE, 2023-2030 (USD MILLION)
- TABLE 288 FRANCE: PREIMPLANTATION GENETIC TESTING MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 289 ITALY: PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 290 ITALY: PREIMPLANTATION GENETIC TESTING MARKET, BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
- TABLE 291 ITALY: PREIMPLANTATION GENETIC TESTING MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 292 ITALY: PREIMPLANTATION GENETIC TESTING MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 293 ITALY: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 294 ITALY: PREIMPLANTATION GENETIC TESTING MARKET, BY TYPE OF CYCLE, 2023-2030 (USD MILLION)
- TABLE 295 ITALY: PREIMPLANTATION GENETIC TESTING MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 296 SPAIN: PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 297 SPAIN: PREIMPLANTATION GENETIC TESTING MARKET, BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
- TABLE 298 SPAIN: PREIMPLANTATION GENETIC TESTING MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 299 SPAIN: PREIMPLANTATION GENETIC TESTING MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 300 SPAIN: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 301 SPAIN: PREIMPLANTATION GENETIC TESTING MARKET, BY TYPE OF CYCLE, 2023-2030 (USD MILLION)
- TABLE 302 SPAIN: PREIMPLANTATION GENETIC TESTING MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 303 REST OF EUROPE: PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 304 REST OF EUROPE: PREIMPLANTATION GENETIC TESTING MARKET, BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
- TABLE 305 REST OF EUROPE: PREIMPLANTATION GENETIC TESTING MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 306 REST OF EUROPE: PREIMPLANTATION GENETIC TESTING MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 307 REST OF EUROPE: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 308 REST OF EUROPE: PREIMPLANTATION GENETIC TESTING MARKET, BY TYPE OF CYCLE, 2023-2030 (USD MILLION)
- TABLE 309 REST OF EUROPE: PREIMPLANTATION GENETIC TESTING MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 310 ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 311 ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 312 ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET, BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
- TABLE 313 ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 314 ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 315 ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 316 ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET, BY TYPE OF CYCLE, 2023-2030 (USD MILLION)
- TABLE 317 ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 318 CHINA: PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 319 CHINA: PREIMPLANTATION GENETIC TESTING MARKET, BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
- TABLE 320 CHINA: PREIMPLANTATION GENETIC TESTING MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 321 CHINA: PREIMPLANTATION GENETIC TESTING MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 322 CHINA: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 323 CHINA: PREIMPLANTATION GENETIC TESTING MARKET, BY TYPE OF CYCLE, 2023-2030 (USD MILLION)
- TABLE 324 CHINA: PREIMPLANTATION GENETIC TESTING MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 325 JAPAN: PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 326 JAPAN: PREIMPLANTATION GENETIC TESTING MARKET, BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
- TABLE 327 JAPAN: PREIMPLANTATION GENETIC TESTING MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 328 JAPAN: PREIMPLANTATION GENETIC TESTING MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 329 JAPAN: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 330 JAPAN: PREIMPLANTATION GENETIC TESTING MARKET, BY TYPE OF CYCLE, 2023-2030 (USD MILLION)
- TABLE 331 JAPAN: PREIMPLANTATION GENETIC TESTING MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 332 INDIA: PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 333 INDIA: PREIMPLANTATION GENETIC TESTING MARKET, BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
- TABLE 334 INDIA: PREIMPLANTATION GENETIC TESTING MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 335 INDIA: PREIMPLANTATION GENETIC TESTING MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 336 INDIA: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 337 INDIA: PREIMPLANTATION GENETIC TESTING MARKET, BY TYPE OF CYCLE, 2023-2030 (USD MILLION)
- TABLE 338 INDIA: PREIMPLANTATION GENETIC TESTING MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 339 AUSTRALIA: PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 340 AUSTRALIA: PREIMPLANTATION GENETIC TESTING MARKET, BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
- TABLE 341 AUSTRALIA: PREIMPLANTATION GENETIC TESTING MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 342 AUSTRALIA: PREIMPLANTATION GENETIC TESTING MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 343 AUSTRALIA: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 344 AUSTRALIA: PREIMPLANTATION GENETIC TESTING MARKET, BY TYPE OF CYCLE, 2023-2030 (USD MILLION)
- TABLE 345 AUSTRALIA: PREIMPLANTATION GENETIC TESTING MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 346 SOUTH KOREA: PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 347 SOUTH KOREA: PREIMPLANTATION GENETIC TESTING MARKET, BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
- TABLE 348 SOUTH KOREA: PREIMPLANTATION GENETIC TESTING MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 349 SOUTH KOREA: PREIMPLANTATION GENETIC TESTING MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 350 SOUTH KOREA: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 351 SOUTH KOREA: PREIMPLANTATION GENETIC TESTING MARKET, BY TYPE OF CYCLE, 2023-2030 (USD MILLION)
- TABLE 352 SOUTH KOREA: PREIMPLANTATION GENETIC TESTING MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 353 REST OF ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 354 REST OF ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET, BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
- TABLE 355 REST OF ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 356 REST OF ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 357 REST OF ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 358 REST OF ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET, BY TYPE OF CYCLE, 2023-2030 (USD MILLION)
- TABLE 359 REST OF ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 360 LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 361 LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 362 LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET, BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
- TABLE 363 LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 364 LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 365 LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 366 LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET, BY TYPE OF CYCLE, 2023-2030 (USD MILLION)
- TABLE 367 LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 368 BRAZIL: PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 369 BRAZIL: PREIMPLANTATION GENETIC TESTING MARKET, BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
- TABLE 370 BRAZIL: PREIMPLANTATION GENETIC TESTING MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 371 BRAZIL: PREIMPLANTATION GENETIC TESTING MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 372 BRAZIL: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 373 BRAZIL: PREIMPLANTATION GENETIC TESTING MARKET, BY TYPE OF CYCLE, 2023-2030 (USD MILLION)
- TABLE 374 BRAZIL: PREIMPLANTATION GENETIC TESTING MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 375 MEXICO: PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 376 MEXICO: PREIMPLANTATION GENETIC TESTING MARKET, BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
- TABLE 377 MEXICO: PREIMPLANTATION GENETIC TESTING MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 378 MEXICO: PREIMPLANTATION GENETIC TESTING MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 379 MEXICO: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 380 MEXICO: PREIMPLANTATION GENETIC TESTING MARKET, BY TYPE OF CYCLE, 2023-2030 (USD MILLION)
- TABLE 381 MEXICO: PREIMPLANTATION GENETIC TESTING MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 382 REST OF LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 383 REST OF LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET, BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
- TABLE 384 REST OF LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 385 REST OF LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 386 REST OF LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 387 REST OF LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET, BY TYPE OF CYCLE, 2023-2030 (USD MILLION)
- TABLE 388 REST OF LATIN AMERICA: PREIMPLANTATION GENETIC TESTING MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 389 MIDDLE EAST: KEY MACROINDICATORS
- TABLE 390 MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 391 MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 392 MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET, BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
- TABLE 393 MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 394 MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 395 MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 396 MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET, BY TYPE OF CYCLE, 2023-2030 (USD MILLION)
- TABLE 397 MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 398 GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 399 GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 400 GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET, BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
- TABLE 401 GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 402 GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 403 GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 404 GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET, BY TYPE OF CYCLE, 2023-2030 (USD MILLION)
- TABLE 405 GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 406 KINGDOM OF SAUDI ARABIA: PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 407 KINGDOM OF SAUDI ARABIA: PREIMPLANTATION GENETIC TESTING MARKET, BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
- TABLE 408 KINGDOM OF SAUDI ARABIA: PREIMPLANTATION GENETIC TESTING MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 409 KINGDOM OF SAUDI ARABIA: PREIMPLANTATION GENETIC TESTING MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 410 KINGDOM OF SAUDI ARABIA: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 411 KINGDOM OF SAUDI ARABIA: PREIMPLANTATION GENETIC TESTING MARKET, BY TYPE OF CYCLE, 2023-2030 (USD MILLION)
- TABLE 412 KINGDOM OF SAUDI ARABIA: PREIMPLANTATION GENETIC TESTING MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 413 UAE: PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 414 UAE: PREIMPLANTATION GENETIC TESTING MARKET, BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
- TABLE 415 UAE: PREIMPLANTATION GENETIC TESTING MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 416 UAE: PREIMPLANTATION GENETIC TESTING MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 417 UAE: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 418 UAE: PREIMPLANTATION GENETIC TESTING MARKET, BY TYPE OF CYCLE, 2023-2030 (USD MILLION)
- TABLE 419 UAE: PREIMPLANTATION GENETIC TESTING MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 420 REST OF GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 421 REST OF GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET, BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
- TABLE 422 REST OF GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 423 REST OF GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 424 REST OF GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 425 REST OF GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET, BY TYPE OF CYCLE, 2023-2030 (USD MILLION)
- TABLE 426 REST OF GCC COUNTRIES: PREIMPLANTATION GENETIC TESTING MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 427 REST OF MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 428 REST OF MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET, BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
- TABLE 429 REST OF MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 430 REST OF MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 431 REST OF MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 432 REST OF MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET, BY TYPE OF CYCLE, 2023-2030 (USD MILLION)
- TABLE 433 REST OF MIDDLE EAST: PREIMPLANTATION GENETIC TESTING MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 434 AFRICA: PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE, 2023-2030 (USD MILLION)
- TABLE 435 AFRICA: PREIMPLANTATION GENETIC TESTING MARKET, BY PROCEDURE TYPE, 2023-2030 (USD MILLION)
- TABLE 436 AFRICA: PREIMPLANTATION GENETIC TESTING MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 437 AFRICA: PREIMPLANTATION GENETIC TESTING MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 438 AFRICA: PREIMPLANTATION GENETIC TESTING MARKET FOR STRUCTURAL CHROMOSOMAL ABNORMALITIES, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 439 AFRICA: PREIMPLANTATION GENETIC TESTING MARKET, BY TYPE OF CYCLE, 2023-2030 (USD MILLION)
- TABLE 440 AFRICA: PREIMPLANTATION GENETIC TESTING MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 441 OVERVIEW OF STRATEGIES DEPLOYED BY KEY PLAYERS IN PREIMPLANTATION GENETIC TESTING MARKET, JANUARY 2022-OCTOBER 2025
- TABLE 442 PREIMPLANTATION GENETIC TESTING MARKET: DEGREE OF COMPETITION
- TABLE 443 PREIMPLANTATION GENETIC TESTING MARKET: REGION FOOTPRINT
- TABLE 444 PREIMPLANTATION GENETIC TESTING MARKET: PRODUCT & SERVICE FOOTPRINT
- TABLE 445 PREIMPLANTATION GENETIC TESTING MARKET: TECHNOLOGY FOOTPRINT
- TABLE 446 PREIMPLANTATION GENETIC TESTING MARKET: APPLICATION FOOTPRINT
- TABLE 447 PREIMPLANTATION GENETIC TESTING MARKET: DETAILED LIST OF KEY STARTUPS/SMES
- TABLE 448 PREIMPLANTATION GENETIC TESTING MARKET: COMPETITIVE BENCHMARKING OF KEY STARTUPS/SMES, BY PRODUCT & SERVICE AND REGION
- TABLE 449 PREIMPLANTATION GENETIC TESTING MARKET: PRODUCT LAUNCHES, JANUARY 2022-OCTOBER 2025
- TABLE 450 PREIMPLANTATION GENETIC TESTING MARKET: DEALS, JANUARY 2022-OCTOBER 2025
- TABLE 451 PREIMPLANTATION GENETIC TESTING MARKET: EXPANSIONS, JANUARY 2022-OCTOBER 2025
- TABLE 452 ILLUMINA, INC.: COMPANY OVERVIEW
- TABLE 453 ILLUMINA, INC.: PRODUCTS/SERVICES/SOLUTIONS OFFERED
- TABLE 454 ILLUMINA, INC.: DEALS, JANUARY 2022-OCTOBER 2025
- TABLE 455 THERMO FISHER SCIENTIFIC INC.: COMPANY OVERVIEW
- TABLE 456 THERMO FISHER SCIENTIFIC INC.: PRODUCTS/SERVICES/SOLUTIONS OFFERED
- TABLE 457 THERMO FISHER SCIENTIFIC INC.: PRODUCT LAUNCHES, JANUARY 2022-OCTOBER 2025
- TABLE 458 AGILENT TECHNOLOGIES, INC.: COMPANY OVERVIEW
- TABLE 459 AGILENT TECHNOLOGIES, INC.: PRODUCTS/SERVICES/SOLUTIONS OFFERED
- TABLE 460 REVVITY: COMPANY OVERVIEW
- TABLE 461 REVVITY: PRODUCTS/SERVICES/SOLUTIONS OFFERED
- TABLE 462 COOPERCOMPANIES: COMPANY OVERVIEW
- TABLE 463 COOPERCOMPANIES: PRODUCTS/SERVICES/SOLUTIONS OFFERED
- TABLE 464 ABBOTT: COMPANY OVERVIEW
- TABLE 465 ABBOTT: PRODUCTS/SERVICES/SOLUTIONS OFFERED
- TABLE 466 TAKARA BIO INC.: COMPANY OVERVIEW
- TABLE 467 TAKARA BIO INC.: PRODUCTS/SERVICES/SOLUTIONS OFFERED
- TABLE 468 QIAGEN: COMPANY OVERVIEW
- TABLE 469 QIAGEN: PRODUCTS/SERVICES/SOLUTIONS OFFERED
- TABLE 470 VITROLIFE: COMPANY OVERVIEW
- TABLE 471 VITROLIFE: PRODUCTS/SERVICES/SOLUTIONS OFFERED
- TABLE 472 OXFORD NANOPORE TECHNOLOGIES PLC: COMPANY OVERVIEW
- TABLE 473 OXFORD NANOPORE TECHNOLOGIES PLC: PRODUCTS/SERVICES/ SOLUTIONS OFFERED
- TABLE 474 OXFORD NANOPORE TECHNOLOGIES PLC: EXPANSIONS, JANUARY 2022-OCTOBER 2025
- TABLE 475 OXFORD GENE TEHNOLOGY IP LIMITED: COMPANY OVERVIEW
- TABLE 476 YIKON GENOMICS: COMPANY OVERVIEW
- TABLE 477 SHIVA SCIENTIFIC: COMPANY OVERVIEW
- TABLE 478 NANJING SUPERYEARS GENE TECHNOLOGY CO., LTD.: COMPANY OVERVIEW
- TABLE 479 MEDICOVER GENETICS: COMPANY OVERVIEW
- TABLE 480 MEDGENOME: COMPANY OVERVIEW
- TABLE 481 FULGENT GENETICS: COMPANY OVERVIEW
- TABLE 482 INVICTA SP. Z O.O.: COMPANY OVERVIEW
- TABLE 483 GENEA PTY LIMITED: COMPANY OVERVIEW
- TABLE 484 SCIGENE CORPORATION: COMPANY OVERVIEW
- TABLE 485 BIOARRAY S.L.: COMPANY OVERVIEW
- TABLE 486 UNIMED BIOTECH (SHANGHAI) CO., LTD.: COMPANY OVERVIEW
- TABLE 487 GENEMIND BIOSCIENCES CO., LTD.: COMPANY OVERVIEW
- TABLE 488 BERRY GENOMICS: COMPANY OVERVIEW
- TABLE 489 BANGKOK GENOMICS INNOVATION: COMPANY OVERVIEW
List of Figures
- FIGURE 1 PREIMPLANTATION GENETIC TESTING MARKET SEGMENTATION & REGIONAL SCOPE
- FIGURE 2 PREIMPLANTATION GENETIC TESTING MARKET: YEARS CONSIDERED
- FIGURE 3 PREIMPLANTATION GENETIC TESTING MARKET, BY PRODUCT & SERVICE, 2025 VS. 2030 (USD MILLION)
- FIGURE 4 PREIMPLANTATION GENETIC TESTING MARKET, BY PROCEDURE TYPE, 2025 VS. 2030 (USD MILLION)
- FIGURE 5 PREIMPLANTATION GENETIC TESTING MARKET, BY TECHNOLOGY, 2025 VS. 2030 (USD MILLION)
- FIGURE 6 PREIMPLANTATION GENETIC TESTING MARKET, BY APPLICATION, 2025 VS. 2030 (USD MILLION)
- FIGURE 7 PREIMPLANTATION GENETIC TESTING MARKET, BY TYPE OF CYCLE, 2025 VS. 2030 (USD MILLION)
- FIGURE 8 PREIMPLANTATION GENETIC TESTING MARKET, BY END USER, 2025 VS. 2030 (USD MILLION)
- FIGURE 9 REGIONAL ANALYSIS: PREIMPLANTATION GENETIC TESTING MARKET
- FIGURE 10 DECLINING FERTILITY RATES TO PROPEL MARKET GROWTH
- FIGURE 11 US AND REAGENTS & CONSUMABLES COMMANDED LARGEST NORTH AMERICAN MARKET SHARE IN 2024
- FIGURE 12 ASIA PACIFIC TO BE FASTEST-GROWING MARKET FROM 2025 TO 2030
- FIGURE 13 REAGENTS & CONSUMABLES TO DOMINATE MARKET DURING STUDY PERIOD
- FIGURE 14 FERTILITY CLINICS ACCOUNTED FOR LARGEST MARKET SHARE IN 2024
- FIGURE 15 PREIMPLANTATION GENETIC TESTING MARKET: DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
- FIGURE 16 PREIMPLANTATION GENETIC TESTING MARKET: TRENDS/DISRUPTIONS IMPACTING CUSTOMER'S BUSINESS
- FIGURE 17 AVERAGE SELLING PRICE TREND OF PREIMPLANTATION GENETIC TESTING INSTRUMENTS, BY KEY PLAYER, 2024 (USD)
- FIGURE 18 AVERAGE SELLING PRICE TREND OF PREIMPLANTATION GENETIC TESTING CONSUMABLES, BY KEY PLAYER, 2024 (USD)
- FIGURE 19 AVERAGE SELLING PRICE TREND OF PREIMPLANTATION GENETIC TESTING INSTRUMENTS, BY REGION, 2024 (USD MILLION)
- FIGURE 20 AVERAGE SELLING PRICE TREND OF PREIMPLANTATION GENETIC TESTING CONSUMABLES, BY REGION, 2024 (USD MILLION)
- FIGURE 21 PREIMPLANTATION GENETIC TESTING MARKET: VALUE CHAIN ANALYSIS
- FIGURE 22 PREIMPLANTATION GENETIC TESTING MARKET: ECOSYSTEM ANALYSIS
- FIGURE 23 PREIMPLANTATION GENETIC TESTING MARKET: PORTER'S FIVE FORCES ANALYSIS
- FIGURE 24 FUNDING AND NUMBER OF DEALS IN PREIMPLANTATION GENETIC TESTING MARKET, 2022-2025 (USD MILLION)
- FIGURE 25 PATENT APPLICATIONS IN PREIMPLANTATION GENETIC TESTING MARKET, JANUARY 2014-DECEMBER 2024
- FIGURE 26 IMPACT OF AI/GEN AI ON PREIMPLANTATION GENETIC TESTING MARKET
- FIGURE 27 US: REGULATORY PROCESS FOR IN VITRO DIAGNOSTIC DEVICES
- FIGURE 28 CANADA: REGULATORY PROCESS FOR IN VITRO DIAGNOSTIC DEVICES
- FIGURE 29 EUROPE: REGULATORY PROCESS FOR IN VITRO DIAGNOSTIC DEVICES
- FIGURE 30 JAPAN: REGULATORY PROCESS FOR IN VITRO DIAGNOSTIC DEVICES
- FIGURE 31 INDIA: REGULATORY PROCESS FOR IN VITRO DIAGNOSTIC DEVICES
- FIGURE 32 BRAZIL: REGULATORY PROCESS FOR IN VITRO DIAGNOSTIC DEVICES
- FIGURE 33 INFLUENCE OF KEY STAKEHOLDERS ON BUYING PROCESS, BY PRODUCT & SERVICE
- FIGURE 34 KEY BUYING CRITERIA FOR MAJOR END USERS
- FIGURE 35 NORTH AMERICA: PREIMPLANTATION GENETIC TESTING MARKET SNAPSHOT
- FIGURE 36 ASIA PACIFIC: PREIMPLANTATION GENETIC TESTING MARKET SNAPSHOT
- FIGURE 37 REVENUE ANALYSIS OF KEY PLAYERS IN PREIMPLANTATION GENETIC TESTING MARKET, 2020-2024 (USD MILLION)
- FIGURE 38 MARKET SHARE ANALYSIS OF KEY PLAYERS IN PREIMPLANTATION GENETIC TESTING MARKET (2024)
- FIGURE 39 EV/EBITDA OF KEY VENDORS
- FIGURE 40 YEAR-TO-DATE (YTD) PRICE, TOTAL RETURN AND 5-YEAR STOCK BETA OF KEY VENDORS
- FIGURE 41 PREIMPLANTATION GENETIC TESTING MARKET: BRAND/PRODUCT COMPARATIVE ANALYSIS
- FIGURE 42 PREIMPLANTATION GENETIC TESTING MARKET: COMPANY EVALUATION MATRIX (KEY PLAYERS), 2024
- FIGURE 43 PREIMPLANTATION GENETIC TESTING MARKET: COMPANY FOOTPRINT
- FIGURE 44 PREIMPLANTATION GENETIC TESTING MARKET: COMPANY EVALUATION MATRIX (STARTUPS/SMES), 2024
- FIGURE 45 ILLUMINA, INC.: COMPANY SNAPSHOT
- FIGURE 46 THERMO FISHER SCIENTIFIC INC.: COMPANY SNAPSHOT
- FIGURE 47 AGILENT TECHNOLOGIES, INC.: COMPANY SNAPSHOT
- FIGURE 48 REVVITY: COMPANY SNAPSHOT
- FIGURE 49 COOPERCOMPANIES: COMPANY SNAPSHOT
- FIGURE 50 ABBOTT: COMPANY SNAPSHOT
- FIGURE 51 TAKARA BIO INC.: COMPANY SNAPSHOT
- FIGURE 52 QIAGEN: COMPANY SNAPSHOT
- FIGURE 53 VITROLIFE: COMPANY SNAPSHOT
- FIGURE 54 OXFORD NANOPORE TECHNOLOGIES PLC: COMPANY SNAPSHOT




