PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1272680

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1272680
Analytical Standards Market - Growth, Trends, and Forecasts (2023 - 2028)
The analytical standards market studied was anticipated to grow with a CAGR of 5.5% during the forecast period.
The emergence of COVID-19 had a significant impact on the studied market. The analytical standard techniques have been extensively used in the manufacturing of the COVID-19 vaccine mainly, thereby contributing to the growth of the market. According to the 2022 update by Agilent technologies, the COVID-19 pandemic led to an increase in research and development (R&D) of vaccines for the disease, and separation-based analytical characterization tools such as chromatography and mass spectrometry were employed by researchers to understand the SARS-CoV-2 virus. For the development of better therapies, vaccines, and diagnostic tools to aid in the fight against the COVID-19 pandemic, the adoption of such analytical methods increased among end users. Thus, the studied market witnessed growth during COVID-19, and it is expected to continue its stronghold during the forecast period.
The major factors attributing to the growth of the analytical standards market are rising stringent quality legislation for drug manufacturers, which has led to a huge demand for analytical standards across the globe. The steep rise in the global prevalence of infectious diseases, HIV, cancers, and other diseases has also necessitated the development of novel drugs and diagnostic products.
The incidence and prevalence of chronic diseases are increasing globally, this is further fueling the demand for drug development by major biopharmaceutical companies. The July 2022 update by the Centers for Disease Prevention and Control (CDC) showed that more than 1 in 7 persons or 15% of adults or 37 million people in the United States had CKD in 2021. Similarly, according to the GLOBOCAN 2020 report, the total number of people in the world affected by cancer is estimated to rise to 28.89 million cases by 2040. The large population affected by chronic diseases and the increase in the necessity of novel drugs generate demand for analytical techniques such as chromatography, spectroscopy, and titrimetry, among others used during drug discovery. Thus, an increasing global burden of diseases leads to develop new drugs, and their validations with enhanced quality led to the growth of the market. Additionally, a steep rise in quality testing of products with regular inspections, certification of analytical procedures, and others are likely to contribute to the growth of the global market during the forecast period.
To make informed decisions concerning the quality and safety of pharmaceutical products, reliable analytical data are required. Such analytical data are also necessary for regulatory submissions supporting the registration of medicinal products. As a result, planning for the anticipated application of the process requires developing meaningful experimental designs that include system suitability characteristics. Thus, manufacturers are focused on the launch of new methods.
For instance, in August 2022, Thermo Fisher Scientific launched a new Raman spectroscopic analyzer for process monitoring for a variety of applications, including biopharmaceutical manufacturing. The Thermo Scientific Ramina Process Analyzer offers non-destructive and continuous analysis without the need for sample preparation, with rapid system setup and deployment in just 15 minutes to generate spectral data on target analytes within seconds. Such developments are majorly contributing to the market growth during the analysis period.
Analytical Standards Market Trends
Chromatography Segment is Expected to Witness Significant Growth
The chromatography segment is anticipated to witness significant growth, among other segments, throughout the forecast period. Due to its high performance in identifying and separating impurities with straightforward procedures, end users adapt the chromatography technique frequently. This is accelerating the growth of the segment and is predicted to continue its growth trend over the analysis period.
The rapid and precise method of separation in various forms of samples contains a wide range of components. Furthermore, the availability of equipment, reagents, and reference standards for various types of chromatography, such as liquid chromatography, gas chromatography, and others, have increased their demand. The large population affected by chronic diseases and the need for novel drugs are a few other factors that contribute to the segment's growth.
Also, the product launches by key market players are expected to drive the growth of the studied segment. For instance, in February 2021, ThermoFisher Scientific launched the Vanquish Online 2D-Liquid Chromatography system, which offers versatility for multidimensional liquid chromatography. In addition, in November 2022, CD Bioparticles launched a range of ion exchange chromatography resins with enhanced loading capacity, ultra-high resolution, and strong mechanical strengths, suitable for the purification of small molecular weight molecules, covering small proteins, polypeptides, nucleic acids, and antibiotics. Such developments are majorly contributing to the market growth during the analysis period.
Additionally, the application of this equipment in-line with other analytical instruments, such as mass spectrometers, etc., increased the adoption of the technique. Thus, easier inter-operability that requires less manpower has reduced the overall analysis cost and is expected to drive the segment's growth.
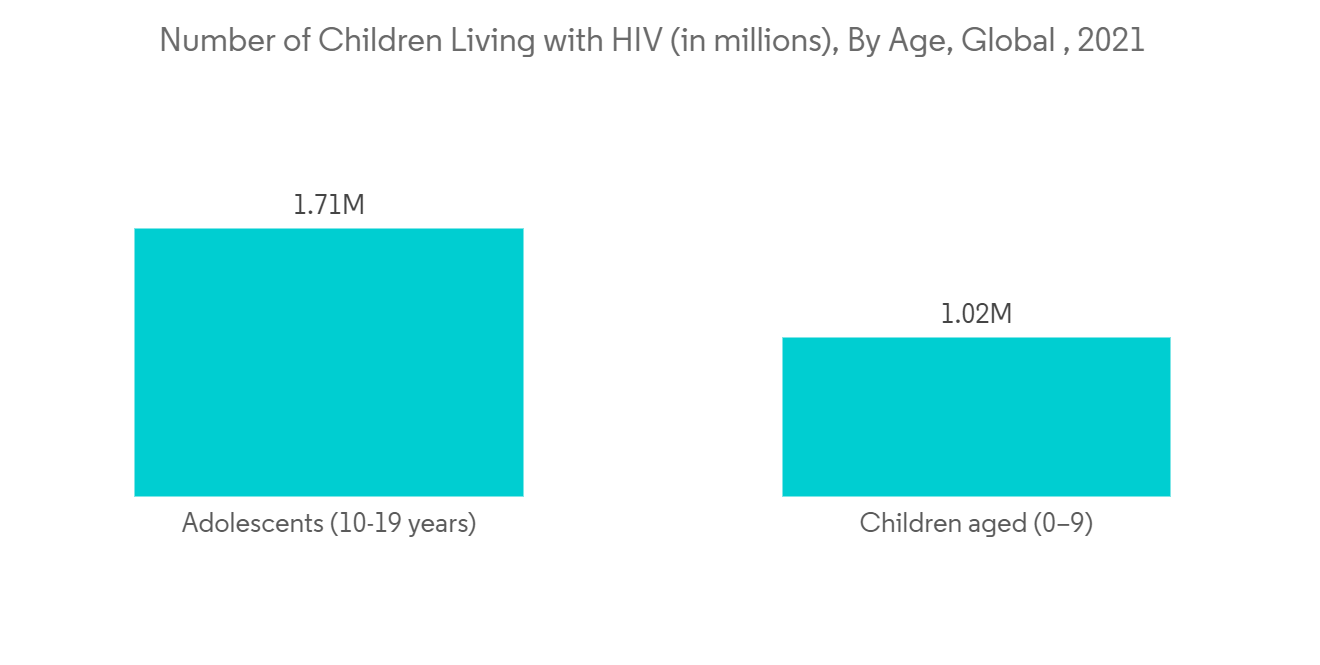
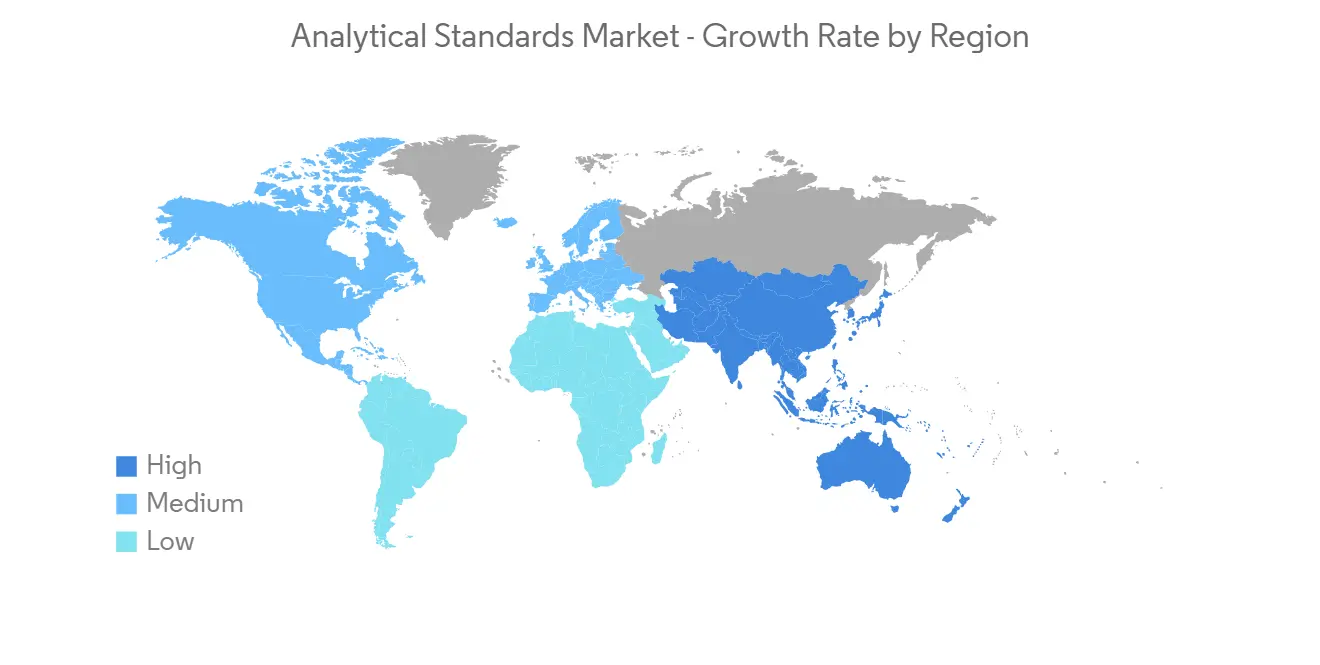
North America is Expected to Hold Largest Market Share
North America will dominate the overall analytical standards market throughout the forecast period. The highest revenue can vary due to the presence of a high volume of key players with higher affordability and favorable government initiatives to develop innovative drugs.
Infectious diseases are a major cause of concern in North America. As per the June 2022 update by CDC, in the United States, young people accounted for 20% (6,135) of all new HIV diagnoses in 2020, while young bisexual men accounted for 84% (5,161) of all new HIV diagnoses in people aged 13 to 24 in the same year. Additionally, Young Black/African American bisexual men are more severely affected and represented 53% (2,740) of new HIV diagnoses among bisexual men in the United States in 2020. This results in the necessity of developing high-quality drugs and other products with enhanced safety precautions in a lesser period. This leads to a rise in demand for analytical standards for new molecules under development. Furthermore, the increased adoption rate of advanced infrastructure by the analytical testing companies and the volume of diagnostic centers are factors estimated to boost the market in the region.
The Canadian market's growth is attributed to the rising burden of a wide range of diseases, an increasing number of clinical trials, rising healthcare expenditure, rising investment in drug development and research, and focus on analytical testing of biologics & biosimilars. As of April 2021, according to ClinicalTrial.gov, Canadian pharmaceutical companies and research institutions have robust R&D pipelines where the pipeline contained 5,659 new studies in various stages of evaluation, 1,397 (24%) were in Phase III clinical trials which were approved through the FDA representing a wide range of therapeutic areas.
Rising healthcare expenditure has been fueling the pharmaceutical analytical testing market growth in the region. Therefore, pharmaceutical companies are shifting to discover novel therapies that require analytical testing during their development. This approach is expected to positively affect pharmaceutical analytical testing sales, thus driving the market growth. Thus, the above-mentioned factors are expected to contribute to the growth of the market in the region during the forecast period.
Analytical Standards Industry Overview
The Analytical standards market is moderately competitive and consists of several major players. Some of the companies which are currently dominating the market are Mallinckrodt Pharmaceuticals, Merck KGaA, Waters Corporation, Restek Corporation, and LGC Group.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Growing Burden of Chronic Diseases Coupled with Increase in Drug Development
- 4.2.2 Increased Demand for Quicker and Reliable Results along with Product Safety and Quality
- 4.3 Market Restraints
- 4.3.1 Lack of Skilled Professionals
- 4.3.2 Challenges in Development of Analytical Standards and Reagents Due to Complex Regulatory Requirements.
- 4.4 Porter Five Forces
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD million)
- 5.1 By Technique
- 5.1.1 Chromatography
- 5.1.2 Spectroscopy
- 5.1.3 Titrimetry
- 5.1.4 Physical Properties Tests
- 5.1.5 Other Techniques
- 5.2 By Product Type
- 5.2.1 Organic
- 5.2.2 Inorganic
- 5.3 By Application
- 5.3.1 Bioanalytical Testing
- 5.3.2 Stability Testing
- 5.3.3 Raw Material Testing
- 5.3.4 Microbial Testing
- 5.3.5 Other Applications
- 5.4 Geography
- 5.4.1 North America
- 5.4.1.1 United States
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 Europe
- 5.4.2.1 Germany
- 5.4.2.2 United Kingdom
- 5.4.2.3 France
- 5.4.2.4 Italy
- 5.4.2.5 Spain
- 5.4.2.6 Rest of Europe
- 5.4.3 Asia-Pacific
- 5.4.3.1 China
- 5.4.3.2 Japan
- 5.4.3.3 India
- 5.4.3.4 Australia
- 5.4.3.5 South Korea
- 5.4.3.6 Rest of Asia-Pacific
- 5.4.4 Middle East and Africa
- 5.4.4.1 GCC
- 5.4.4.2 South Africa
- 5.4.4.3 Rest of Middle East and Africa
- 5.4.5 South America
- 5.4.5.1 Brazil
- 5.4.5.2 Argentina
- 5.4.5.3 Rest of South America
- 5.4.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Spex Certiprep (Antylia Scientific)
- 6.1.2 Waters Corporation
- 6.1.3 Mallinckrodt Pharmaceuticals
- 6.1.4 Restek Corporation
- 6.1.5 Merck KGaA
- 6.1.6 LGC Group
- 6.1.7 GFS Chemicals Inc.
- 6.1.8 Chiron AS
- 6.1.9 Agilent Technologies Inc.
- 6.1.10 Cytiva (Danaher)
7 MARKET OPPORTUNITIES AND FUTURE TRENDS




