Need help finding what you are looking for?
Contact Us
PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1403131

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1403131
France Spinal Surgery Devices - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts 2024 - 2029
PUBLISHED:
PAGES: 70 Pages
DELIVERY TIME: 2-3 business days
SELECT AN OPTION
The French spinal surgery devices market is expected to register a CAGR of 5.13% during the forecast period.
Key Highlights
- COVID-19 impacted spinal surgery devices owing to the cancellations of elective surgeries and outpatient clinics in the country in its initial phase. However, the country was soon able to manage spinal surgeries despite pandemic restrictions. For instance, an article published in Elsevier Public Health Emergency Collection in October 2021 stated that spinal units in France were able to provide a good standard of care despite the worldwide pandemic for patients who required urgent and non-deformable spinal surgery. Therefore, the spinal devices market regained growth in the later phase of the pandemic. In the current scenario, COVID-19 had a negligible impact on the market since restrictions have been lifted.
- The condition known as rheumatoid arthritis (RA) is common among the population of France. For instance, according to the research study published in June 2022 in BMC Primary Care Journal, RA is one of the most common chronic and inflammatory diseases in rheumatology. Its prevalence is estimated at 0.35% every year in France. This creates a disease burden in the country. Spinal surgery provides considerable improvements in the treatment of RA. Thus, the high burden of RA is expected to demand the availability of spinal surgery devices for treatment purposes, further accelerating the studied market growth in France.
- Furthermore, the geriatric population is likely to increase the market growth over the forecast period. For instance, according to the 2022 statistics published by the United Nations Population Fund, in France, a large proportion of the population is expected to be aged 15-64 years and accounts for 61% in 2022. In addition, as per the same source, 21% of the population was aged 65 years and above in 2022. Thus, the rising geriatric populations are more prone to develop diseases such as arthritis and spinal deformity, which require spinal surgical procedures, thereby boosting the market growth over the forecast period.
- Additionally, the strategic initiatives by market players for expanding spinal surgery devices in the country are also expected to propel market growth. For instance, in September 2022, E Cential Robotics, a France-based company specializing in surgical robotics, developed a spine surgery platform that provides intra-operative 2D and 3D imaging, navigation, and robotic guidance for performing surgical procedures. The platform's surgical arm was granted 510(k) clearance from the United States Food and Drug Administration (US FDA) in September 2022 and received 510(k) approval for the platform's imaging system in 2021. Therefore, the rising approvals and launches by market players are expected to accelerate market growth.
- Therefore, owing to the aforementioned factors, the studied market is anticipated to witness growth over the analysis period. However, the stringent reimbursement concerns and expensive treatment procedures are likely to impede market growth.
France Spinal Surgery Devices Market Trends
Arthroplasty Devices Segment is Expected to Hold Significant Share in the French Spinal Surgery Devices Market
- Arthroplasty is a surgical procedure to restore the function of a joint. Arthroplasty is a type of orthopedic surgery in which the articular surface of a musculoskeletal joint is replaced, remodeled, or realigned through osteotomy or another procedure. The segment is expected to grow due to the high burden of arthroplasty procedures in the country, as well as technological advancements.
- The strategic initiatives by market players, such as product launches and expansions, are also contributing to the segment's growth. For instance, in March 2022, Exactech, a developer and producer of innovative implants, instrumentation, and smart technologies for joint replacement surgery, launched Equinoxe Humeral Reconstruction Prosthesis for clinical use in Europe, including France. The humeral reconstruction prosthesis is used in primary or revision arthroplasty procedures.
- Furthermore, in August 2022, Exactech conducted one of the first successful Spartan Stem and Logical Cup System surgeries for total hip arthroplasty. Therefore, the device's rising launches and success rate are expected to boost the segment's growth, accelerating the market growth.
- Therefore, rising product launches are expected to propel the segment's growth during the forecast period.
Fracture Repair Devices is Expected to Register Healthy Growth Over the Forecast Period
- The fracture repair devices are expected to register healthy growth due to the increasing burden of spine diseases and fractures as a result of musculoskeletal disorders. Spinal cord injury stems from a traumatic blow on the spine that fractures, dislocates, crushes, or compresses one or more vertebrae. Therefore, spinal cord injuries boost the demand for fracture repair devices for treating such conditions. For instance, an article published in Medecine Intensive Reanimation in September 2021 stated that the number of spinal cord injuries in France is around 2000 new cases per year. The high burden of spinal cord injury propels the segment's growth during the forecast period.
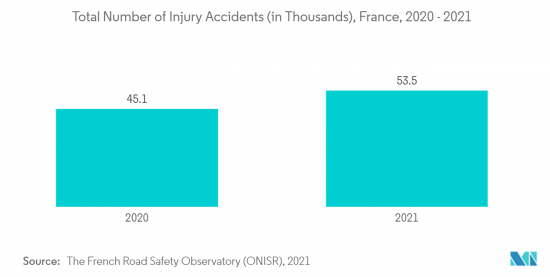
- Furthermore, per the International Osteoporosis Foundation 2021 update, the new fragility fractures are estimated to increase by 26.0% in 2034, representing 610,000 fractures in 2034. Additionally, according to the French Road Safety Observatory (French: Observatoire National Interministeriel de la Securite Routiere (ONISR)) 2023 update, there were 53,540 injury traffic accidents and 67,057 injured people during the accidents in mainland France in 2021. Osteoporosis is reported to complicate spinal surgery; hence, the high burden of osteoporosis and road accidents is anticipated to increase the spinal fractures and augment the utility for fracture repair devices, propelling the segment's growth during the forecast period.
France Spinal Surgery Devices Industry Overview
The French spinal surgery devices market is competitive and consists of several players. In terms of market share, a few of the major players are currently dominating the market, and some prominent players are vigorously making acquisitions and joint ventures with other companies to consolidate their market positions in the country. Some key market players are Zimmer Biomet, Stryker Corporation, Johnson & Johnson, Medtronic PLC, and Globus Medical.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
Product Code: 49780
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Rise in Technological Advances in Spinal Surgery and Increasing Demand for Minimally Invasive Surgical Procedures
- 4.2.2 Increasing Incidence of Obesity, Aging Population, and Associated Spine Disorders
- 4.3 Market Restraints
- 4.3.1 Expensive Treatment Procedures and Stringent Reimbursement Concerns
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD million)
- 5.1 By Device Type
- 5.1.1 Spinal Decompression
- 5.1.1.1 Corpectomy
- 5.1.1.2 Discectomy
- 5.1.1.3 Facetectomy
- 5.1.1.4 Foraminotomy
- 5.1.1.5 Laminotomy
- 5.1.2 Spinal Fusion
- 5.1.2.1 Cervical Fusion
- 5.1.2.2 Interbody Fusion
- 5.1.2.3 ThoracoLumbar Fusion
- 5.1.2.4 Other Spinal Fusions
- 5.1.3 Fracture Repair Devices
- 5.1.4 Arthroplasty Devices
- 5.1.5 Non-fusion Devices
- 5.1.6 Other Fracture Repair Devices
- 5.1.1 Spinal Decompression
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Zimmer Biomet
- 6.1.2 Stryker Corporation
- 6.1.3 Johnson & Johnson
- 6.1.4 Medtronic PLC
- 6.1.5 Globus Medical
- 6.1.6 SeaSpine Holdings Corporation
- 6.1.7 Orthofix Holdings Inc.
- 6.1.8 Nuvasive Inc.
- 6.1.9 Joimax GmbH
- 6.1.10 RTI Surgical Holdings Inc.
7 MARKET OPPORTUNITIES AND FUTURE TRENDS
Have a question?


SELECT AN OPTION
Have a question?


Questions? Please give us a call or visit the contact form.
