PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1440182

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1440182
Japan Pharmaceutical - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2024 - 2029)
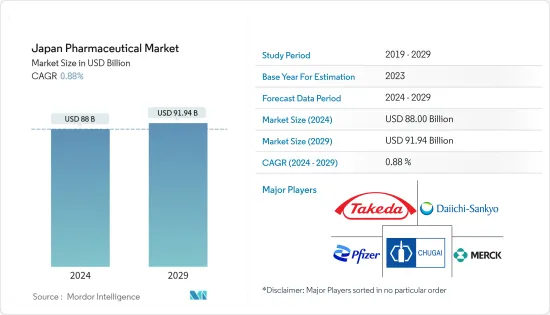
The Japan Pharmaceutical Market size is estimated at USD 88 billion in 2024, and is expected to reach USD 91.94 billion by 2029, growing at a CAGR of 0.88% during the forecast period (2024-2029).
The COVID-19 pandemic significantly impacted the pharmaceutical market in Japan. The increased COVID-19 infection cases in the country increased the demand for prescription drugs and vaccines, which impacted the demand for pharmaceutical products. The increasing import of COVID-19 vaccines increased the demand for pharmaceutical products. For instance, in May 2021, the Japanese government signed a contract with Pfizer-BioNTech to import 194 million doses of vaccine by the end of 2021. The Japanese government granted emergency approval to COVID-19 vaccines and drugs, which impacted the market's growth. For instance, in May 2020, remdesivir was approved by the country to treat COVID-19 patients, while a vaccine was granted approval in February 2021. With the relaxation of pandemic-related restrictions and resuming company activities in manufacturing drugs and other products, the pharmaceutical market is expected to grow over the forecast period.
Factors such as the rising geriatric population, increasing incidence of chronic diseases, and growing research and development (R&D) investments in the country are boosting the market's growth in Japan.
The rising number of infections and chronic diseases such as cardiovascular diseases, diabetes, hypertension, cancer, neurological diseases, and others are driving the market's growth. According to the GLOBOCAN 2020 report, 1,028,658 new cancer cases were reported in Japan in 2020, and the total number of five-year prevalent cancer cases was 2,710,728. The same report projected the number of cancer cases to reach 1,110,549 by 2030 and 1,128,057 by 2040 in Japan. Thus, the expected increase in the number of people suffering from cancer is anticipated to increase the demand for effective drugs, boosting the market's growth.
An article published in May 2021 observed that 6.5 to 7 million people are expected to be living with dementia by 2025. About 8.5 to 11.5 million people in Japan are expected to have dementia by 2060. Thus, the expected increase in the number of people suffering from dementia is anticipated to increase the demand for drugs used to treat the disease. This is anticipated to fuel the market's growth during the forecast period.
The increasing geriatric population in the country is contributing to the market's growth. According to the 2022 statistics published by UNPF, 59% of the total population living in Japan is aged between 15-64 in 2022. As per the same source, 29% of the population is aged 65 years and above in 2022. Thus, the rising geriatric population is more prone to develop chronic diseases, such as cardiovascular diseases, neurological disorders, and cancer, raising the demand for effective therapeutics, which is expected to boost the market's growth.
The increasing focus on research and development activities and rising research and health expenditure in the country are expected to increase the development and availability of pharmaceutical products, bolstering the market's growth. For instance, as per 2021 statistics published by the OECD, Japan spent 3.27% of its GDP on its R&D activities in 2020.
In Japan, two crucial regulatory bodies review and approve drugs and medical devices: PMDA and MHLW. The regulatory committees engage in monitoring and surveillance to ensure the safety and efficacy of authorized biologics or pharmaceuticals in Japan. According to an article published in April 2021, the drug approval process in Japan is less complex and easy compared to some other countries. The PMDA and regulatory aspects offer consultation to sponsors to help them understand the requirements and the stepwise drug approval process. Hence, the number of companies is increasing in Japan, which is anticipated to increase the development of drugs, thereby propelling the market's growth.
The increasing investments and advances in the field of pharmaceuticals are increasing in the country. This is anticipated to drive market growth. For instance, in May 2022, ExoCoBio Inc. received a Japanese patent claiming that stem cell-derived exosome is an effective ingredient for alleviating dermatitis. The patent results from the vigorous R&D efforts to develop therapeutic medicines with higher efficacy and safety than currently known ones for dermatitis with itching and inflammation. Also, in March 2022, Japan launched a new research and development center for USD 1.6 billion to support the vaccine and drug projects as a part of a larger scheme to tackle infectious diseases. The establishment of an R&D facility for innovative partnerships and budget management will be under Supervisory Control And Data Acquisition (SCARDA). Thus, such developments are anticipated to drive market growth in Japan.
However, stringent regulatory scenarios for several products are expected to hinder the market's growth during the forecast period.
Japan Pharmaceutical Market Trends
Prescription Drugs Segment is Expected to Hold a Significant Share in the Market Over Forecast Period
The prescription drugs segment is expected to witness significant growth in the pharmaceutical market over the forecast period due to factors such as the rising prevalence of chronic diseases, the increasing geriatric population, the growing demand and adoption of prescription drugs, and increasing product launches in the country.
The rising number of people suffering chronic diseases in Japan is increasing the demand for the development of pharmaceutical products such as vaccines, biologics, or other therapeutic drugs, hence boosting the market's growth. According to the WHO, as of August 9, 2022, 14,421,539 confirmed cases of COVID-19 and 33,825 deaths were reported in Japan. Thus, the increasing number of COVID-19 cases among the population is expected to increase the demand for COVID-19 vaccines in the country, thereby propelling the market's growth.
The rising company focus on developing pharmaceutical products and adopting various business strategies such as collaboration, partnerships, and product launches are also contributing to the growth of the market studied. For instance, in March 2022, the Japanese Ministry of Health, Labor, and Welfare (MHLW) granted marketing authorization for Xenpozyme (olipudase alfa) for the treatment of adult and pediatric patients with non-central nervous system (non-CNS) manifestations of acid sphingomyelinase deficiency (ASMD). In January 2022, Chugai's anti-IL-6 receptor monoclonal antibody Actemra was approved for the additional indication of the treatment of SARS-CoV-2 pneumonia (limited to patients requiring oxygen intervention) in Japan.
Therefore, due to the above-mentioned factors, the prescription drugs segment is expected to hold a significant market share in the Japanese pharmaceutical market over the forecast period.
Respiratory System Segment is Expected to Have a Significant Market Share Over the Forecast Period
The respiratory segment is expected to grow over the forecast period due to the increasing prevalence of respiratory diseases, such as asthma, chronic obstructive respiratory diseases, and others. The rising pediatric and aging population is more susceptible to developing respiratory diseases due to weak immunity, which is also contributing to the market's growth. According to the GLOBOCAN 2020 report, 138,532 new lung cancer cases were reported in Japan in 2020, and this number is projected to double by 2040. According to an article published in the Journal of Thoracic Disease in June 2021, the prevalence of chronic obstructive pulmonary disease (COPD) was higher among the population aged 40 years and above. Thus, the high burden of respiratory diseases among the population is anticipated to increase the demand for asthma and COPD drugs, propelling the market's growth.
The rising company activities in developing drugs and increasing drug approvals are expected to increase the market's growth. For instance, in September 2022, the Japanese MHLW approved AstraZeneca's Tezspire (tezepelumab) for the treatment of bronchial asthma in patients with severe or refractory disease in whom asthma symptoms cannot be controlled with mid- or high-dose inhaled corticosteroids and other long-term maintenance therapies, in Japan. In August 2022, the Japanese MHLW approved AstraZeneca's Tagrisso (osimertinib) for the adjuvant treatment of patients with epidermal growth factor receptor mutated (EGFRm) non-small cell lung cancer (NSCLC) after surgery in Japan.
In May 2022, the Japanese MHLW approved Chugai Pharmaceutical's Tecentriq for an additional indication of the anti-cancer agent/humanized anti-PD-L1 monoclonal antibody for the adjuvant treatment of PD-L1-positive non-small cell lung cancer (NSCLC). In January 2022, the Japanese MHLW approved Amgen's Lumakras for the treatment of KRAS G12C-mutated positive, unresectable, advanced, and recurrent non-small cell lung cancer (NSCLC) that has progressed after systemic anti-cancer therapy in Japan.
Therefore, due to the above-mentioned factors, the respiratory system segment is expected to grow during the forecast period.
Japan Pharmaceutical Industry Overview
The competitive landscape of the Japanese pharmaceutical market covers the business overview, financials, products, and strategies followed by major companies. The pharmaceutical market in Japan is highly competitive and consists of several major players. In terms of market share, a few major players are dominating the market studied. Some prominent players are vigorously making acquisitions and joint ventures with other companies to consolidate their market positions in the country. Some key companies currently dominating the market are Takeda Pharmaceutical Company Limited, Pfizer Inc., Chugai Pharmaceutical Co. Ltd, Merck & Co. Inc., and Daiichi Sankyo Company, Limited, among others.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.1.1 Healthcare Expenditure
- 4.1.2 Pharmaceutical Imports and Exports
- 4.1.3 Epidemiology Data For key Diseases
- 4.1.4 Regulatory Landscape/Regulatory Bodies
- 4.1.5 Licensing and Market Authorization
- 4.1.6 Pipeline Analysis
- 4.1.6.1 By Phase
- 4.1.6.2 By Sponsor
- 4.1.6.3 By Disease
- 4.1.7 Statically Overview
- 4.1.7.1 Number of Hospitals
- 4.1.7.2 Employment in the Pharmaceutical Sector
- 4.1.7.3 R&D Expenditure
- 4.1.8 Ease of Doing Business
- 4.2 Market Drivers
- 4.2.1 Rising Geriatric Population and Increasing Burden of Chronic Diseases
- 4.2.2 Increasing Research and Development Activities Along with Growing R&D Investments
- 4.3 Market Restraints
- 4.3.1 Stringent Regulatory Scenario
- 4.4 Porter's Five Force Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD million)
- 5.1 By Therapeutic Category
- 5.1.1 Antiallergics
- 5.1.2 Blood and Blood Forming Organs
- 5.1.3 Cardiovascular System
- 5.1.4 Dermatologicals
- 5.1.5 Genito Urinary System
- 5.1.6 Respiratory System
- 5.1.7 Sensory Organs
- 5.1.8 Other Therapeutic Categories
- 5.2 By Prescription Type
- 5.2.1 Prescription Drugs
- 5.2.1.1 Branded
- 5.2.1.2 Generics
- 5.2.2 OTC Drugs
- 5.2.1 Prescription Drugs
6 COMPETITIVE LANDSCAPE AND COMPANY PROFILES
- 6.1 Company Profiles
- 6.1.1 Takeda Pharmaceutical Company Limited
- 6.1.2 Astellas Pharma Inc.
- 6.1.3 Pfizer Inc.
- 6.1.4 Chugai Pharmaceutical Co. Ltd
- 6.1.5 Merck & Co. Inc.
- 6.1.6 Daiichi Sankyo Company Limited
- 6.1.7 Bayer AG
- 6.1.8 GlaxoSmithKline PLC
- 6.1.9 Eli Lilly and Company
- 6.1.10 Novartis International AG
- 6.1.11 Sanofi SA
- 6.1.12 Catalent Inc.
- 6.1.13 Eisai Co. Ltd
- 6.1.14 Johnson and Johnson (Janssen Global Services)
- 6.1.15 Aspen Holdings
7 MARKET OPPORTUNITIES AND FUTURE TRENDS




