PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1835642

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1835642
Hairy Cell Leukemia - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
The hairy cell leukemia treatment market stands at USD 125.26 million in 2025 and is forecast to advance to USD 167.32 million by 2030, reflecting a 5.96% CAGR throughout the period.
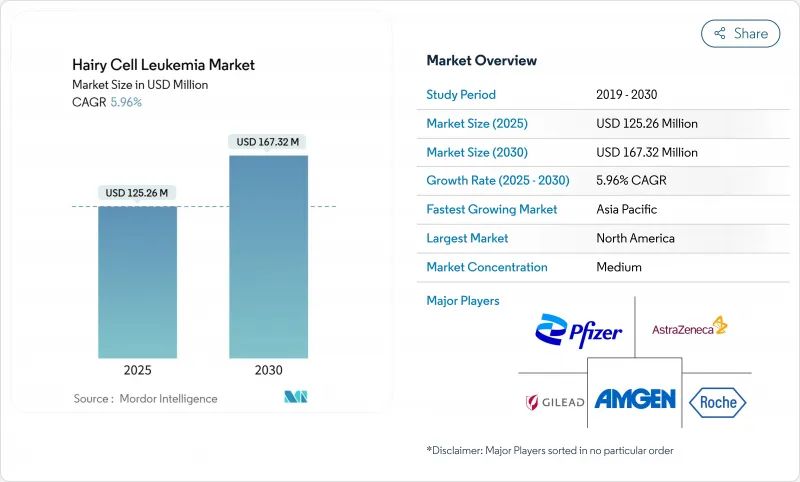
The upward curve emerges from rapid uptake of precision therapies that directly target the BRAF V600E mutation, early diagnostic confirmation through flow cytometry and molecular panels, and the continued regulatory commitment to orphan-drug innovation by the FDA and EMA. Improved life expectancy among treated patients intensifies the need for long-term monitoring solutions, while broader telemedicine coverage moves specialist knowledge beyond academic centers to underserved geographies. Competitive activity focuses on combination protocols that couple purine analogues with monoclonal antibodies or kinase inhibitors, aligning efficacy with tolerability. Meanwhile, oral and subcutaneous formulations challenge the dominance of infusion-center care, fostering home-based treatment pathways that lower systemic costs.
Global Hairy Cell Leukemia Market Trends and Insights
Growing Burden of Leukemia Cases & Higher Diagnosis Rates
Flow cytometry and immunophenotyping now uncover cases that previously slipped under clinical radar, raising confirmed incidence counts and enabling prompt therapeutic intervention. AI-enabled pattern recognition archives a 97.5% concordance with manual reads while trimming processing time by 60.3%, increasing throughput and standardizing quality. Telemedicine links provincial laboratories with metropolitan reference centers, accelerating second opinions for atypical findings. Refinement of morphological scoring has also clarified epidemiology; the near-universal BRAF V600E hallmark provides a single-gene anchor that removes diagnostic ambiguity. These advances collectively swell treatment-eligible populations and shorten lead times to first treatment.
Rising Geriatric Population
Median presentation age remains between 50 and 55 years, yet prevalence rises sharply in octogenarian cohorts. Bone marrow biopsy audits in individuals aged 85 plus revised initial diagnoses in 44.1% and reshaped therapy plans in 25.4% without added complications. Older patients push demand for regimens that offer deep responses without myelosuppressive intensity, tilting prescribing toward BRAF or BTK inhibition. Subcutaneous dosing schedules reduce hospital stays, align with mobility constraints, and thus resonate with senior lifestyle preferences. Prolonged post-remission survival further necessitates structured surveillance and retreatment triggers.
Limited Awareness & Specialist Access in Rural Areas
Resource limitations confine immunophenotyping and molecular labs to tertiary centers. Case series from low-income regions reveal splenectomy still substituted for cladribine because supply chains do not consistently stock purine analogues. Hematologist shortages persist, with 113 radiation oncologists serving 110 million citizens in the Philippines, a ratio that illustrates systemic gaps. Tele-oncology mitigates but does not yet erase these disparities, as patchy internet coverage curtails video consultation reliability in remote districts.
Other drivers and restraints analyzed in the detailed report include:
- Rapid Uptake of Next-Generation Targeted Therapies
- MRD-Guided Retreatment Algorithms
- High Cost of Novel Targeted Agents
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Targeted modalities register the swiftest momentum, as the segment grows at an 8.56% CAGR to 2030. Near-universal BRAF V600E positivity provides a crisp biomarker, and the vemurafenib-rituximab pair delivers 96% complete response, cementing proof-of-concept. BTK inhibition furnishes an alternative axis for relapsed or mutation-resistant subsets, and early-line integration trials are underway. Immunotoxin therapy such as CD22-directed moxetumomab pasudotox remains reserved for multiply relapsed disease because of capillary leak risk and price considerations.
Chemotherapy upholds a powerful footprint, owning 61.45% of hairy cell leukemia treatment market share in 2024. Cladribine and pentostatin continue as frontline standards thanks to 80% plus complete remission rates and decades of clinician familiarity. Rituximab co-administration elevates depth of response while curbing relapse frequency, sustaining chemotherapy relevance. Emerging subcutaneous formulations support outpatient or home deployment, fending off share erosion.
The Hairy Cell Leukemia Market is Segmented by Therapy Type (Chemotherapy, Targeted Therapy, Immunotherapy, and Others), Patient Type (Classic HCL, Variant HCL, and SDRPL & Other HCL-Like Disorders), Route of Administration (Intravenous Infusion, Subcutaneous Injection, and Oral) and Geography (North America, Europe, Asia-Pacific, Middle East and Africa, and South America). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America steered 42.34% of 2024 global sales, anchored by dense networks of specialty hematology centers that facilitate rapid adoption of innovative regimens and real-time MRD surveillance. FDA decisions continue to influence worldwide standard-setting, with breakthrough labels cutting development timelines from years to months. Tele-oncology services have grown rapidly; the Mayo Clinic reports oncology virtual-care completion rates above 90%, broadening specialist reach.
Asia-Pacific is set to grow at an 8.93% CAGR owing to expanding tertiary care capacity and policy frameworks that encourage clinical trial hosting. Chinese leukemia incidence stabilizes, yet survival metrics improve as technology diffusion continues. India's updated trial guidelines now align with ICH-GCP, accelerating start-up timelines and enhancing safety governance. This ecosystem invites multinational sponsors to enroll previously unserved patients, bolstering early access.
Europe benefits from the EMA's centralized approval mechanism, permitting simultaneous market entry across member states once reimbursement dossiers clear national HTA bodies. Pan-regional cooperative research groups sustain high enrollment for investigator-initiated trials, particularly those comparing novel kinase inhibitors against purine analog benchmarks.
Latin America, the Middle East, and Africa record gradual uptake, yet supply chain gaps and reimbursement constraints hinder pace. Splenectomy persists in certain low-resource pockets, reflecting therapy access shortfalls. International partnerships that supply subsidized drug, diagnostic kits, and telepathology teaching promise to narrow disparity margins over the coming decade.
- Roche
- Pfizer
- AstraZeneca
- Gilead Sciences
- Amgen
- Johnson & Johnson
- Merck
- Merck
- Bristol-Myers Squibb
- Novartis
- Abbvie
- Innate Pharma SA
- Genmab
- BeiGene
- Adaptive Biotechnologies Corp.
- ADC Therapeutics
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Growing burden of leukemia cases & higher diagnosis rates
- 4.2.2 Rising geriatric population
- 4.2.3 Rapid uptake of next-generation targeted therapies
- 4.2.4 MRD-guided retreatment algorithms
- 4.2.5 Home-based sub-cutaneous cladribine protocols
- 4.2.6 Support of Regulatory Authorities
- 4.3 Market Restraints
- 4.3.1 Limited awareness & specialist access in rural areas
- 4.3.2 High cost of novel targeted agents
- 4.3.3 Orphan-drug exclusivity expiries post-2028
- 4.3.4 Severe immunosuppression & infection risk with purine analogues
- 4.4 Value / Supply-Chain Analysis
- 4.5 Regulatory Landscape
- 4.6 Technology Outlook
- 4.7 Porter's Five Forces Analysis
- 4.7.1 Bargaining Power of Suppliers
- 4.7.2 Bargaining Power of Buyers
- 4.7.3 Threat of New Entrants
- 4.7.4 Threat of Substitutes
- 4.7.5 Intensity of Competitive Rivalry
5 Market Size and Growth Forecasts (Value-USD)
- 5.1 By Therapy Type
- 5.1.1 Chemotherapy (purine analogues)
- 5.1.2 Targeted Therapy (BRAF, BTK, MEK inhibitors)
- 5.1.3 Immunotherapy (mAbs, immunotoxins)
- 5.1.4 Others (interferon-a, splenectomy)
- 5.2 By Patient Type
- 5.2.1 Classic HCL (cHCL)
- 5.2.2 Variant HCL (HCL-V)
- 5.2.3 SDRPL & other HCL-like disorders
- 5.3 By Route of Administration
- 5.3.1 Intravenous Infusion
- 5.3.2 Sub-cutaneous Injection
- 5.3.3 Oral
- 5.4 By Geography
- 5.4.1 North America
- 5.4.1.1 United States
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 Europe
- 5.4.2.1 Germany
- 5.4.2.2 United Kingdom
- 5.4.2.3 France
- 5.4.2.4 Italy
- 5.4.2.5 Spain
- 5.4.2.6 Rest of Europe
- 5.4.3 Asia-Pacific
- 5.4.3.1 China
- 5.4.3.2 Japan
- 5.4.3.3 India
- 5.4.3.4 Australia
- 5.4.3.5 South Korea
- 5.4.3.6 Rest of Asia-Pacific
- 5.4.4 Middle East and Africa
- 5.4.4.1 GCC
- 5.4.4.2 South Africa
- 5.4.4.3 Rest of Middle East and Africa
- 5.4.5 South America
- 5.4.5.1 Brazil
- 5.4.5.2 Argentina
- 5.4.5.3 Rest of South America
- 5.4.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products and Services, and Recent Developments)
- 6.3.1 F. Hoffmann-La Roche Ltd
- 6.3.2 Pfizer Inc.
- 6.3.3 AstraZeneca plc
- 6.3.4 Gilead Sciences Inc.
- 6.3.5 Amgen Inc.
- 6.3.6 Johnson & Johnson (Janssen)
- 6.3.7 Merck KGaA
- 6.3.8 Merck & Co., Inc.
- 6.3.9 Bristol-Myers Squibb Co.
- 6.3.10 Novartis AG
- 6.3.11 AbbVie Inc.
- 6.3.12 Innate Pharma SA
- 6.3.13 Genmab A/S
- 6.3.14 BeiGene Ltd
- 6.3.15 Adaptive Biotechnologies Corp.
- 6.3.16 ADC Therapeutics SA
7 Market Opportunities and Future Outlook
- 7.1 White-Space and Unmet-Need Assessment




