PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1836458

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1836458
Vein Illuminator - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
The Vein Illuminator Market size is estimated at USD 0.3 billion in 2025, and is expected to reach USD 1.04 billion by 2030, at a CAGR of 27.80% during the forecast period (2025-2030).
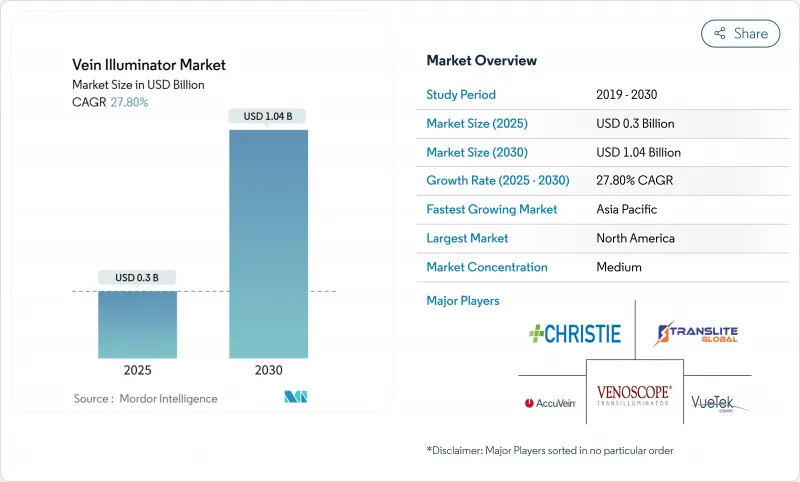
Robust growth reflects health systems' focus on first-attempt venipuncture success, an outcome now tied to U.S. Medicare's value-based purchasing scores. Demand is amplified by aging and obese populations that make traditional vein palpation unreliable, while rising chronic-disease monitoring requires more frequent blood draws. Technology improvements in near-infrared (NIR) imaging, falling component costs, and portable form factors further accelerate adoption. Asia-Pacific's push to localize medical-device manufacturing and China's hospital modernization are tilting future revenue toward cost-optimized systems. Competitive pressure is intensifying as local firms introduce low-price NIR devices that undercut established brands while premium models layer on AI guidance and multi-modal imaging.
Global Vein Illuminator Market Trends and Insights
Rising First-Attempt Success Rates Drive Quality Metrics
Clinical trials in pediatric units showed first-stick success climbing to 74.1% with AccuVein AV400 compared with 40.7% using palpation, trimming procedure time from 169 seconds to 44 seconds. Health-system executives translate these gains directly into higher HCAHPS patient-experience scores, which shape Medicare reimbursements, elevating device purchases to strategic priorities. Patient surveys reveal 93% of respondents rate hospitals higher when staff employ visualization tools.
Growth in Chronic-Disease Blood Draws
More frequent HbA1c, lipid, and renal tests among diabetic and cardiovascular cohorts raise annual venipuncture volumes, stressing phlebotomy capacity. Aging vasculature and drug-induced vein fragility heighten failure risk, prompting facilities to equip labs with portable NIR finders that cut repeat sticks and consumable waste.
High Capital and Per-Unit Device Costs
Premium NIR systems list between USD 4,000 and USD 27,000, squeezing budgets of small hospitals. Experimental open-source models built from recycled optics have demonstrated comparable vein contrast for USD 25, hinting at future price erosion.
Other drivers and restraints analyzed in the detailed report include:
- Ageing and Obese Populations Challenge Traditional Methods
- Hospital Push for Patient-Experience KPIs
- Lack of Reimbursement Codes
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Near-Infrared Illumination controlled 50.3% revenue in 2024, underpinning the vein illuminator market with a mature, cost-efficient platform. Ultrasound-Augmented units, posting 31.8% CAGR to 2030, carve share in difficult-access patients via deeper imaging and synergy with existing ultrasound carts. Transillumination remains a pediatric niche due to softer light, while multispectral hybrids gain research traction. Patent filings such as the dual-mode VeinCAP system illustrate convergence trends toward single devices offering NIR plus diffuse hyperspectral views. As feature sets widen, vendors differentiate on AI algorithms that auto-grade vein quality and log success metrics to electronic health records.
Hand-held and Portable devices occupied 61.2% of 2024 revenue because nurses favor pocketable tools that shift easily between wards. Wearable and Clip-On Modules, climbing at 34.1% CAGR, free clinicians' hands during complex cannulations, and feed video to smart glasses for teaching. Table-top carts persist in blood banks where mounted cameras stay calibrated for long draws. IoT connectivity is redefining design priorities: next-generation wearables integrate Wi-Fi and cloud dashboards that benchmark first-stick rates, transforming basic lights into quality-management nodes.
Vein Illuminator Market is Segmented by Technology (Near-Infrared (NIR) Illumination, Transillumination, and More), Product Type (Hand-Held and Portable, Table-Top/Cart-Mounted, and Wearable and Clip-On Modules), Application (Intravenous (IV) Access, Blood Draw/Venipuncture Assistance, and More), End-User (Hospitals and Clinics, Blood Donation Camps and Blood Banks, and More), and Geography.
Geography Analysis
North America retained 37.2% 2024 revenue leadership on the back of sophisticated infrastructure and reimbursement programs that pay for patient-experience outcomes. U.S. hospitals embed first-stick statistics into quality dashboards, ensuring repeat device orders. Canada's single-payer system favors province-wide contracts that lower per-unit costs, while Mexico's private medical-tourism clinics install finders as patient-comfort differentiators.
Europe's multi-payer environment produces steady uptake; Germany's university hospitals pilot multi-modal units, and the United Kingdom's NHS negotiates bulk pricing to support vascular-access safety goals. CE Mark harmonization smooths cross-border sales and encourages newer entrants from Scandinavia and Eastern Europe.
The vein illuminator market size in Asia-Pacific is expanding at a 33.2% CAGR, making it the global growth engine. India's Production-Linked Incentive scheme subsidizes domestic device plants, reducing import reliance. China's hospital-upgrade program requires equipment that boosts nursing efficiency; local brands undercut imports by bundling visualization with IV kits. Japan's super-aged population and high device standards favor premium dual-mode systems, while South Korea's start-ups test AI-enabled smartphone adapters for home infusion services.
- AccuVein Inc.
- Christie Medical Holdings Inc.
- TransLite LLC (Veinlite)
- VueTek Scientific LLC
- Venoscope LLC
- Near Infrared Imaging Inc.
- ZD Medical Inc.
- VeinSight (Surmount Electronic)
- Shenzhen Vivolight Medical Device and Technology Co.
- Veincas Medical Ltd.
- SIFSOF LLC
- NextVein LLC
- Infinium Medical Inc.
- B. Braun Medical Inc.
- Koninklijke Philips N.V.
- Siemens Healthineers AG
- Baxter International Inc.
- GE Healthcare Technologies Inc.
- Osang Healthcare Co., Ltd.
- Kingmaker Biomedical Inc.
- Dhanika Instrument Co.
- Zhongke Micro-Light Medical Equipment Co.
- YSENMED Medical Equipment Co.
- Xavant Medical (Pty) Ltd.
- ALEH Medical Laser and Systems
- FY Medical Devices Co.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET LANDSCAPE
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Rising first-attempt success rates for IV and phlebotomy
- 4.2.2 Growth in chronic-disease related blood draws
- 4.2.3 Ageing and obese populations with difficult venous access
- 4.2.4 Hospital push for patient-experience KPIs
- 4.2.5 AI-integrated mobile vein-finder apps
- 4.2.6 Adoption in cosmetic/aesthetic injections
- 4.3 Market Restraints
- 4.3.1 High capital and per-unit device costs
- 4.3.2 Lack of reimbursement codes
- 4.3.3 Training gaps in low-resource settings
- 4.3.4 Regulatory ambiguity for aesthetic-only devices
- 4.4 Industry Value Chain Analysis
- 4.5 Regulatory Landscape
- 4.6 Technological Outlook
- 4.7 Industry Attractiveness - Porter's Five Forces Analysis
- 4.7.1 Bargaining Power of Suppliers
- 4.7.2 Bargaining Power of Buyers
- 4.7.3 Threat of New Entrants
- 4.7.4 Threat of Substitutes
- 4.7.5 Intensity of Competitive Rivalry
- 4.8 Impact of Macroeconomic Factors on the Market
5 MARKET SIZE AND GROWTH FORECASTS (VALUES)
- 5.1 By Technology
- 5.1.1 Near-Infrared (NIR) Illumination
- 5.1.2 Transillumination
- 5.1.3 Ultrasound-Augmented
- 5.1.4 Multispectral/Hybrid
- 5.1.5 Others
- 5.2 By Product Type
- 5.2.1 Hand-held and Portable
- 5.2.2 Table-Top/Cart-Mounted
- 5.2.3 Wearable and Clip-On Modules
- 5.3 By Application
- 5.3.1 Intravenous (IV) Access
- 5.3.2 Blood Draw/Venipuncture Assistance
- 5.3.3 Sclerotherapy and Varicose Vein Treatment
- 5.3.4 Emergency and Critical Care
- 5.3.5 Cosmetic/Aesthetic Injections
- 5.4 By End-user
- 5.4.1 Hospitals and Clinics
- 5.4.2 Blood Donation Camps and Blood Banks
- 5.4.3 Ambulatory Surgical Centers
- 5.4.4 Rehabilitation and Nursing Homes
- 5.4.5 Academic and Research Institutions
- 5.5 By Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.2 South America
- 5.5.2.1 Brazil
- 5.5.2.2 Argentina
- 5.5.2.3 Chile
- 5.5.2.4 Rest of South America
- 5.5.3 Europe
- 5.5.3.1 Germany
- 5.5.3.2 United Kingdom
- 5.5.3.3 France
- 5.5.3.4 Italy
- 5.5.3.5 Spain
- 5.5.3.6 Russia
- 5.5.3.7 Rest of Europe
- 5.5.4 Asia-Pacific
- 5.5.4.1 China
- 5.5.4.2 India
- 5.5.4.3 Japan
- 5.5.4.4 South Korea
- 5.5.4.5 Malaysia
- 5.5.4.6 Singapore
- 5.5.4.7 Australia
- 5.5.4.8 Rest of Asia-Pacific
- 5.5.5 Middle East and Africa
- 5.5.5.1 Middle East
- 5.5.5.1.1 United Arab Emirates
- 5.5.5.1.2 Saudi Arabia
- 5.5.5.1.3 Turkey
- 5.5.5.1.4 Rest of Middle East
- 5.5.5.2 Africa
- 5.5.5.2.1 South Africa
- 5.5.5.2.2 Nigeria
- 5.5.5.2.3 Rest of Africa
- 5.5.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Market Concentration
- 6.2 Strategic Moves
- 6.3 Market Share Analysis
- 6.4 Company Profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products and Services, and Recent Developments)
- 6.4.1 AccuVein Inc.
- 6.4.2 Christie Medical Holdings Inc.
- 6.4.3 TransLite LLC (Veinlite)
- 6.4.4 VueTek Scientific LLC
- 6.4.5 Venoscope LLC
- 6.4.6 Near Infrared Imaging Inc.
- 6.4.7 ZD Medical Inc.
- 6.4.8 VeinSight (Surmount Electronic)
- 6.4.9 Shenzhen Vivolight Medical Device and Technology Co.
- 6.4.10 Veincas Medical Ltd.
- 6.4.11 SIFSOF LLC
- 6.4.12 NextVein LLC
- 6.4.13 Infinium Medical Inc.
- 6.4.14 B. Braun Medical Inc.
- 6.4.15 Koninklijke Philips N.V.
- 6.4.16 Siemens Healthineers AG
- 6.4.17 Baxter International Inc.
- 6.4.18 GE Healthcare Technologies Inc.
- 6.4.19 Osang Healthcare Co., Ltd.
- 6.4.20 Kingmaker Biomedical Inc.
- 6.4.21 Dhanika Instrument Co.
- 6.4.22 Zhongke Micro-Light Medical Equipment Co.
- 6.4.23 YSENMED Medical Equipment Co.
- 6.4.24 Xavant Medical (Pty) Ltd.
- 6.4.25 ALEH Medical Laser and Systems
- 6.4.26 FY Medical Devices Co.
7 MARKET OPPORTUNITIES AND FUTURE TRENDS
- 7.1 White-Space and Unmet-Need Assessment




