PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1836532

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1836532
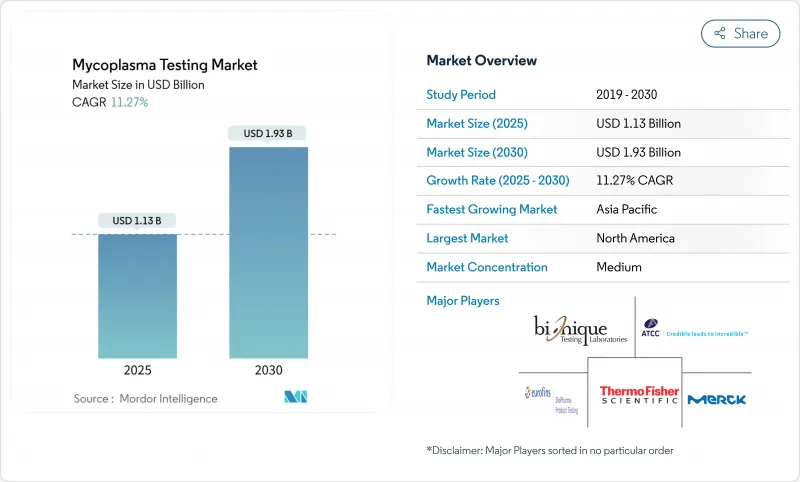
Mycoplasma Testing - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
The Mycoplasma Testing Market size is estimated at USD 1.13 billion in 2025, and is expected to reach USD 1.93 billion by 2030, at a CAGR of 11.27% during the forecast period (2025-2030).
Heightened regulatory scrutiny on biologics manufacturing, expanding cell- and gene-therapy production, and growing preference for rapid nucleic-acid methods underpin this trajectory. Regulatory agencies, led by the FDA and EMA, require validated mycoplasma detection at multiple points in the product lifecycle, turning compliance into a non-discretionary spend for biomanufacturers fda.gov. Digital PCR and automated sample-to-answer platforms accelerate lot-release timelines, while the outsourcing trend transfers testing workloads to contract organizations that can scale capacity quickly. Regional manufacturing expansions in China, India, and Singapore combine with tax incentives to spur laboratory build-outs, yet shortages of skilled molecular QA personnel and high automation costs temper adoption rates in smaller facilities. Competitive dynamics favor vendors able to bundle instruments, kits, and services, boosting cross-selling potential and consolidating customer relationships.
Global Mycoplasma Testing Market Trends and Insights
Expansion of Biopharma and Cell & Gene Therapy Manufacturing Facilities
Capacity ramp-ups in autologous and allogeneic therapy plants have multiplied sample volumes that require 100% batch testing. Contamination prevalence in cell cultures ranges between 15% and 35%, and FDA guidance now mandates mycoplasma testing after pooling and prior to washing, increasing sampling frequency. Continuous perfusion bioreactors raise real-time monitoring needs, pushing demand for automated analyzers that handle higher throughputs with minimal human intervention. Facility roll-outs in emerging markets must complete qualification studies, creating greenfield demand for third-party test services. Together, these trends add 2.8 percentage points to the forecast CAGR.
Regulatory Mandates Require Mycoplasma Release Testing for Biologics
The FDA's laboratory developed tests rule and EMA guidance for advanced therapy medicinal Products require validated assays from a master cell bank to a finished drug. The European Medicines Agency's guidelines for Advanced Therapy Medicinal Products mandate comprehensive mycoplasma testing throughout the manufacturing process, from master cell banks to final product release. These regulatory changes create non-discretionary demand for testing services, as manufacturers cannot release products without demonstrating mycoplasma absence through validated methods. Compliance spending accelerates through 2027 as phased LDT oversight takes effect.The phased implementation of new LDT regulations over four years creates predictable demand growth as laboratories upgrade their testing capabilities to maintain compliance.
High Capital Expenditure for Implementing Automated Detection Systems
Entry-level automated analyzers cost USD 100,000-500,000, with annual service contracts topping USD 50,000. Smaller CTLs and academic labs struggle to justify such outlays, prolonging reliance on manual culture methods. FDA LDT compliance adds validation costs and strains capital budgets, curbing near-term uptake. Smaller contract testing laboratories face particular challenges in justifying automation investments given their limited sample volumes and diverse testing requirements. The complexity of validating automated systems for regulatory compliance adds significant time and cost burdens, with validation studies typically requiring 6-12 months and specialized expertise.
Other drivers and restraints analyzed in the detailed report include:
- Increasing Incidents of Cell-Culture Contamination
- Rising Demand for Rapid, High-Sensitivity PCR-Based Assays
- False-Positive/-Negative Retest Delays
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Kits and reagents maintained a 46.17% share in 2024, underlining their consumable nature within the Mycoplasma testing market. The Services segment is set to expand at 14.68% CAGR as biomanufacturers delegate method validation and routine screening to accredited laboratories. High regulatory hurdles and evolving assay formats encourage companies to buy expert capacity instead of building it. Eurofins' network of 45+ global sites illustrates how scale produces cost-efficiencies that individual firms cannot replicate.
Automation-ready instruments show steady but slower growth because purchasers often tie them to long-term reagent contracts. The BIOFIRE platform and Rapid Micro Biosystems' vial-reader appeal by blending speed with pharmacopeial compliance. As services grow, kit vendors align with third-party labs under reagent-rental models that lock in supply revenues. This synergy consolidates buyer-supplier dependencies and drives volume in both categories.
qPCR holds 64.39% of the 2024 Mycoplasma testing market share thanks to entrenched protocols and broad instrument availability. Digital PCR, with a 16.26% growth rate, mitigates standard-curve errors and detects rare events vital for gene-therapy lots. Sensitivity gains resonate with regulatory auditors seeking robust quantification. Conventional PCR remains a budget choice for legacy facilities, while ELISA and DNA staining stay relevant in niche academic applications. Next-generation sequencing promises multiplex pathogen screens but awaits regulatory consensus.
Regulatory bodies now accept nucleic-acid methods equivalent to culture for release testing, catalysing digital PCR uptake. Instrument makers invest in microfluidic chip formats that slice reaction mixes into thousands of partitions, lowering detection thresholds. AI augmentation further lessens operator skill barriers, unlocking broader lab adoption.
The Mycoplasma Testing Market Report Segments the Industry Into by Product (Instruments, Kits and Reagents, Services), Technology (Conventional PCR, QPCR, and More), Application (Cell-Line Quality Control, and More), End User (Biopharma and Biotechnology Companies, and More), and Geography (North America, Europe, Asia-Pacific, Middle East and Africa, South America). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America led with 40.81% of 2024 revenue as FDA oversight, mature bioprocessing infrastructure, and early technology adoption underpin stable demand. The region's biocluster density and capital availability encourage rapid replacement of culture methods with automated PCR systems. Service providers leverage proximity to innovators, enabling just-in-time sample logistics and compliance audits.
Europe follows with a cohesive regulatory framework from the EMA and harmonised pharmacopoeias that facilitate multi-country lot release. The Mycoplasma testing market size tied to EU gene-therapy trials rises as Germany, Spain, and the United Kingdom host GMP facilities. Vendors cater to multi-language documentation and serialization demands, spurring informatics-enabled assay platforms.
Asia-Pacific represents the fastest-growing arena, expanding at 18.62% CAGR. China's cell-therapy sector benefits from government priority listings, while India's production-linked incentive schemes attract vaccine exporters. Singapore's decentralized QC labs shorten turnaround times for regional biologics plants. Fragmented regulations necessitate local validation, favouring global companies that co-locate service hubs.
South America and the Middle East & Africa trail in absolute revenue but offer untapped upside as domestic vaccine programs and biosimilar plants proliferate. Logistics hurdles and limited cold-chain infrastructure slow penetration of rapid PCR devices, yet public-health investments could unlock future orders once training and service networks mature.
- Thermo Fisher Scientific
- Charles River Laboratories Intl. Inc.
- Merck
- Lonza Group Ltd.
- Eurofins
- Sartorius
- Agilent Technologies
- ATCC
- PromoCell
- Minerva Biolabs GmbH
- bioMerieux
- Bionique Testing Laboratories
- WuXi App Tec
- SGS
- Danaher
- Rapid Micro Biosystems
- MicroBioLogics Inc.
- InvivoGen
- Mycoplasma Experience Ltd.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Expansion of Biopharma and Cell & Gene Therapy (CGT) Manufacturing Facilities
- 4.2.2 Regulatory Mandates Require Mycoplasma Release Testing for Biologics
- 4.2.3 Increasing Incidents of Cell-Culture contamination
- 4.2.4 Rising Demand for Rapid, High-Sensitivity PCR-Based Assays
- 4.2.5 Decentralised QC Labs in Emerging Biotech Hubs
- 4.2.6 Venture Capital-Backed Proliferation of Synthetic Biology Startups
- 4.3 Market Restraints
- 4.3.1 High Capital Expenditure (CAPEX) for Implementing Automated Detection Systems
- 4.3.2 False-Positive/-Negative Retest Delays
- 4.3.3 Skilled Molecular-QA Workforce Shortages
- 4.3.4 Regulatory Lag Surrounding Approval of Microfluidic and Next-Gen Assays
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitutes
- 4.4.5 Intensity of Competitive Rivalry
5 Market Size & Growth Forecasts (Value in USD)
- 5.1 By Product & Service
- 5.1.1 Instruments
- 5.1.1.1 Real-time PCR Systems
- 5.1.1.2 Rapid Microfluidic Analyzers
- 5.1.1.3 Automated Detection Platforms
- 5.1.1.4 Other Instruments
- 5.1.2 Kits & Reagents
- 5.1.2.1 PCR Assay Kits
- 5.1.2.2 ELISA Kits
- 5.1.2.3 Enzymatic Assay Kits
- 5.1.2.4 Fluorescent-Staining Reagents
- 5.1.2.5 Others
- 5.1.3 Services
- 5.1.1 Instruments
- 5.2 By Technology
- 5.2.1 Conventional PCR
- 5.2.2 qPCR
- 5.2.3 Digital PCR
- 5.2.4 ELISA
- 5.2.5 Enzymatic Methods
- 5.2.6 DNA Staining
- 5.2.7 Next-Generation Sequencing
- 5.2.8 Other NAATs
- 5.3 By Application
- 5.3.1 Cell-Line Quality Control
- 5.3.2 Bioproduction Batch-Release Testing
- 5.3.3 Raw-Material & Media Testing
- 5.3.4 Gene & Cell Therapy Manufacturing
- 5.3.5 Vaccine & Virus Testing
- 5.3.6 Other Applications
- 5.4 By End User
- 5.4.1 Biopharma & Biotechnology Companies
- 5.4.2 Contract Manufacturing Organizations (CMOs)
- 5.4.3 Academic & Research Institutes
- 5.4.4 Cell Banks & Repositories
- 5.4.5 Diagnostic & Reference Laboratories
- 5.5 By Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.2 Europe
- 5.5.2.1 Germany
- 5.5.2.2 United Kingdom
- 5.5.2.3 France
- 5.5.2.4 Italy
- 5.5.2.5 Spain
- 5.5.2.6 Rest of Europe
- 5.5.3 Asia-Pacific
- 5.5.3.1 China
- 5.5.3.2 Japan
- 5.5.3.3 India
- 5.5.3.4 Australia
- 5.5.3.5 South Korea
- 5.5.3.6 Rest of Asia-Pacific
- 5.5.4 Middle East & Africa
- 5.5.4.1 GCC
- 5.5.4.2 South Africa
- 5.5.4.3 Rest of Middle East & Africa
- 5.5.5 South America
- 5.5.5.1 Brazil
- 5.5.5.2 Argentina
- 5.5.5.3 Rest of South America
- 5.5.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company Profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products & Services, and Recent Developments)
- 6.3.1 Thermo Fisher Scientific Inc.
- 6.3.2 Charles River Laboratories Intl. Inc.
- 6.3.3 Merck KGaA
- 6.3.4 Lonza Group Ltd.
- 6.3.5 Eurofins Scientific SE
- 6.3.6 Sartorius AG
- 6.3.7 Agilent Technologies
- 6.3.8 ATCC
- 6.3.9 PromoCell GmbH
- 6.3.10 Minerva Biolabs GmbH
- 6.3.11 bioMerieux SA
- 6.3.12 Bionique Testing Laboratories Inc.
- 6.3.13 WuXi AppTec
- 6.3.14 SGS SA
- 6.3.15 Danaher Corporation
- 6.3.16 Rapid Micro Biosystems
- 6.3.17 MicroBioLogics Inc.
- 6.3.18 InvivoGen
- 6.3.19 Mycoplasma Experience Ltd.
7 Market Opportunities & Future Outlook
- 7.1 White-space & Unmet-Need Assessment




