PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1836686

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1836686
Nerve Repair And Regeneration - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
The nerve repair and regeneration market is valued at USD 10.65 billion in 2025 and is projected to reach USD 18.94 billion in 2030, reflecting a 12.20% CAGR over the forecast period.
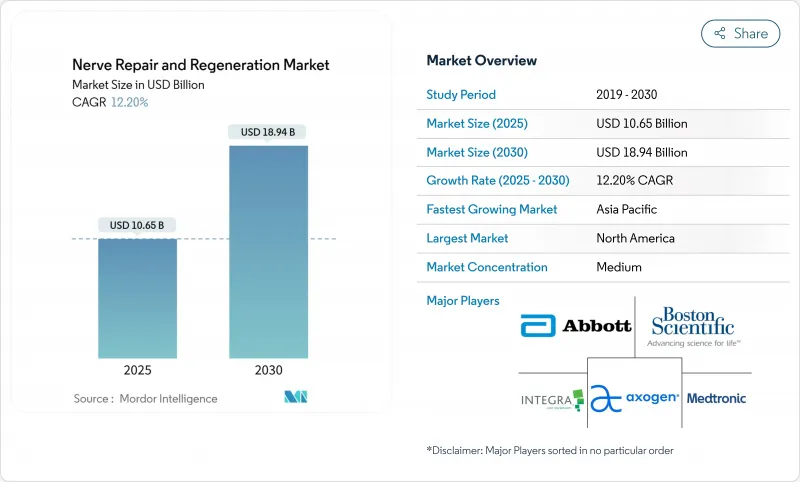
Continued progress in bioelectronic medicine, the growing burden of neurological disorders, and supportive public funding anchor this expansion. AI-enabled closed-loop neurostimulation, 3D-bioprinted patient-specific nerve grafts, and real-time brain-signal monitoring systems are reshaping clinical practice from reactive procedures to precision-guided regeneration. Intensifying adoption of these innovations reveals fresh opportunities across both device and biomaterial categories inside the nerve repair and regeneration market. Demand is reinforced by a demographic shift toward aging populations that experience more diabetes-linked peripheral neuropathies and by increasing battlefield and industrial traumas that require advanced reconstruction therapies. Robust reimbursement in North America, large untapped patient pools in Asia-Pacific, and deep venture funding for emerging biotechnology firms together underpin the global growth outlook.
Global Nerve Repair And Regeneration Market Trends and Insights
Growing Incidence Of Nerve Injuries & Neurological Disorders
Upper-extremity nerve injuries affect 43.8 per million people annually in the United States, with average charges of USD 47,004 per case. Diabetes-linked peripheral neuropathy and age-related neurodegeneration further enlarge the candidate pool for regenerative interventions. The burden extends beyond acute trauma to long-term disability, pushing health systems to embrace earlier nerve reconstruction. Military conflicts and industrial accidents keep peripheral nerve trauma on policy agendas, while better understanding of nerve pathophysiology broadens eligibility for advanced therapies. Collectively these patterns expand the addressable population in the nerve repair and regeneration market.
Technological Advances In Neuro-Modulation & Biomaterials
Medtronic's Percept RC neurostimulator captures real-time brain signals and personalizes therapy delivery. Closed-loop control represents a shift from static to dynamic intervention, potentially improving outcomes and lowering adverse events. Concurrently, 3D-bioprinted chitosan conduits embedded with neurotrophin-3 create bionic microenvironments for peripheral repair. Conductive silk-fibroin scaffolds combined with electrical stimulation have surpassed traditional guides in preclinical recovery metrics. These breakthroughs position biomaterials as a regeneration-first alternative, signalling a major product-mix shift inside the nerve repair and regeneration market.
High Cost Of Implants & Procedures
Spinal cord stimulators range from USD 20,000 to USD 50,000 per implant, excluding surgical expenses, and lifetime costs can surpass USD 100,000 even in insured regions. Supply chain shortages in medical-grade components have inflated prices and elongated lead times. High up-front costs dampen early adoption in low-resource settings and slow diffusion of advanced technologies in the nerve repair and regeneration market.
Other drivers and restraints analyzed in the detailed report include:
- Rising Healthcare Expenditure & Favourable Reimbursement
- AI-Enabled Closed-Loop Bio-Electronic Medicine Adoption
- Cyber-Security & Data-Privacy Risks In Connected Implants
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Neurostimulation and neuromodulation devices accounted for 59.35% of the nerve repair and regeneration market share in 2024, powered by extensive clinical evidence, surgeon familiarity, and established reimbursement channels. Within the same year, biomaterials began reshaping demand patterns through scaffold-free conduits derived from autologous fibroblasts that passed early human safety benchmarks. The nerve repair and regeneration market size tied to biomaterials is expected to grow at 14.25% CAGR, riding on 3D-printing that fabricates patient-specific grafts and chitosan conduits delivering controlled neurotrophic factors.
Internal neurostimulators dominate revenue with higher average selling prices and preferred indications like chronic neuropathic pain, Parkinson's disease, and spinal injury. External stimulators, including transcranial magnetic and trans-cutaneous electrical devices, post steady uptake in rehabilitation therapy. Conductive silk-fibroin scaffolds loaded with gold nanoparticles are yielding superior axonal growth in preclinical work, hinting at future substitution potential for traditional leads. As cost curves of biofabrication decline and clinical data matures, biomaterials are poised to transform long-term product mix inside the nerve repair and regeneration market.
The Nerve Repair and Regeneration Market Report is Segmented by Product Type (Neurostimulation and Neuromodulation Devices, and Biomaterials), Application (Neurostimulation and Neuromodulation Surgeries, Direct Nerve Repair / Neurorrhaphy, and More), End-User (Hospitals, Ambulatory Surgical Centres, and More), and Geography (North America, Europe, Asia-Pacific, and More). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America retained 41.82% revenue share in 2024 on the back of advanced insurance coverage, deep clinical trial density, and continuous public R&D investment such as the USD 2.833 billion NINDS budget. Military research through DARPA's Bridging the Gap Plus program and the USD 650 million Military Burn Research Program further accelerates innovation in peripheral nerve repair. Canada adds incremental growth via universal health benefits that support equitable access, and Mexico improves cross-border procedure volumes through medical tourism packages. Cybersecurity regulation from the FDA shapes device certification standards and influences global export success.
Asia-Pacific is forecast to deliver a 12.61% CAGR, the highest regional pace, propelled by large patient pools and active government promotion of brain-computer interfaces. China's National Healthcare Security Administration formally recognized neural care services, paving the way for scaled reimbursement. Japan contributes sophisticated engineering and an aging demographic with high neurological disease prevalence. India advances through private hospital expansion and lower-cost procedural pricing that attract regional medical tourism. Australia's world-first olfactory ensheathing cell trial positions the country as a translational research hub.
Europe maintains solid share through coordinated healthcare systems and device adoption. Germany leverages industrial design strengths, while the United Kingdom spearheads early-stage stem-cell studies. Regulatory harmonization under the Medical Device Regulation streamlines continental approvals, speeding dissemination of next-gen implants. Middle East and Africa begin scaling high-acuity centers in urban corridors, though limited specialist availability caps penetration. South America exhibits steady improvements as Brazil and Argentina allocate more budget to neurological care, ensuring that the nerve repair and regeneration market continues its global diffusion.
- Medtronic
- Boston Scientific
- Abbott Laboratories
- Stryker
- Integra LifeSciences
- LivaNova
- Axogen
- Baxter
- Neuronetics
- NeuroPace
- Phagenesis
- MyndTec
- CheckPoint Surgical
- Synapse Biomedical
- Polyganics
- Renishaw
- Collagen Matrix
- BlueWind Medical
- ElectroCore
- Stimwave
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Growing Incidence Of Nerve Injuries & Neurological Disorders
- 4.2.2 Technological Advances In Neuro-Modulation & Biomaterials
- 4.2.3 Rising Healthcare Expenditure & Favourable Reimbursement
- 4.2.4 AI-Enabled Closed-Loop Bio-Electronic Medicine Adoption
- 4.2.5 Military & Elite-Sports Funding For Peripheral-Nerve Repair
- 4.2.6 3-D Bioprinted, Patient-Specific Nerve Graft Breakthroughs
- 4.3 Market Restraints
- 4.3.1 High Cost Of Implants & Procedures
- 4.3.2 Dearth Of Trained Neurosurgeons & Rehab Specialists
- 4.3.3 Medical-Grade Polymer (Chitosan, PTFE) Supply Constraints
- 4.3.4 Cyber-Security & Data-Privacy Risks In Connected Implants
- 4.4 Porter's Five Forces
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitutes
- 4.4.5 Competitive Rivalry
5 Market Size & Growth Forecasts (Value, USD)
- 5.1 By Product Type
- 5.1.1 Neurostimulation & Neuromodulation Devices
- 5.1.1.1 Internal Neurostimulation Devices
- 5.1.1.1.1 Spinal Cord Stimulation (SCS)
- 5.1.1.1.2 Deep Brain Stimulation (DBS)
- 5.1.1.1.3 Vagus Nerve Stimulation (VNS)
- 5.1.1.1.4 Sacral Nerve Stimulation (SNS)
- 5.1.1.1.5 Gastric Electrical Stimulation (GES)
- 5.1.1.2 External Neurostimulation Devices
- 5.1.1.2.1 Trans-cutaneous Electrical Nerve Stimulation (TENS)
- 5.1.1.2.2 Trans-cranial Magnetic Stimulation (TMS)
- 5.1.2 Biomaterials
- 5.1.2.1 Nerve Conduits
- 5.1.2.2 Nerve Protectors
- 5.1.2.3 Nerve Connectors
- 5.1.2.4 Other Biomaterials
- 5.1.1 Neurostimulation & Neuromodulation Devices
- 5.2 By Application
- 5.2.1 Neuro-stimulation & Neuro-modulation Surgeries
- 5.2.2 Direct Nerve Repair / Neurorrhaphy
- 5.2.3 Nerve Grafting
- 5.2.4 Stem-Cell Therapy
- 5.2.5 Other Applications
- 5.3 By End-User
- 5.3.1 Hospitals
- 5.3.2 Ambulatory Surgical Centres
- 5.3.3 Specialty Neurology & Orthopaedic Clinics
- 5.3.4 Rehabilitation Centres
- 5.4 Geography
- 5.4.1 North America
- 5.4.1.1 United States
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 Europe
- 5.4.2.1 Germany
- 5.4.2.2 United Kingdom
- 5.4.2.3 France
- 5.4.2.4 Italy
- 5.4.2.5 Spain
- 5.4.2.6 Rest of Europe
- 5.4.3 Asia-Pacific
- 5.4.3.1 China
- 5.4.3.2 Japan
- 5.4.3.3 India
- 5.4.3.4 South Korea
- 5.4.3.5 Australia
- 5.4.3.6 Rest of Asia-Pacific
- 5.4.4 Middle East and Africa
- 5.4.4.1 GCC
- 5.4.4.2 South Africa
- 5.4.4.3 Rest of Middle East and Africa
- 5.4.5 South America
- 5.4.5.1 Brazil
- 5.4.5.2 Argentina
- 5.4.5.3 Rest of South America
- 5.4.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company Profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products & Services, and Recent Developments)
- 6.3.1 Medtronic plc
- 6.3.2 Boston Scientific Corp.
- 6.3.3 Abbott Laboratories
- 6.3.4 Stryker
- 6.3.5 Integra LifeSciences Corporation
- 6.3.6 LivaNova
- 6.3.7 Axogen Corporation
- 6.3.8 Baxter
- 6.3.9 Neuronetics
- 6.3.10 NeuroPace
- 6.3.11 Phagenesis
- 6.3.12 MyndTec
- 6.3.13 CheckPoint Surgical
- 6.3.14 Synapse Biomedical
- 6.3.15 Polyganics
- 6.3.16 Renishaw
- 6.3.17 Collagen Matrix
- 6.3.18 BlueWind Medical
- 6.3.19 ElectroCore
- 6.3.20 Stimwave
7 Market Opportunities & Future Outlook
- 7.1 White-space & Unmet-Need Assessment




