PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1836705

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1836705
Anti-Snoring Devices - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
The anti-snoring treatment market reached USD 2.04 billion in 2025 and is forecast to advance to USD 3.37 billion by 2030, registering a 10.6% CAGR.
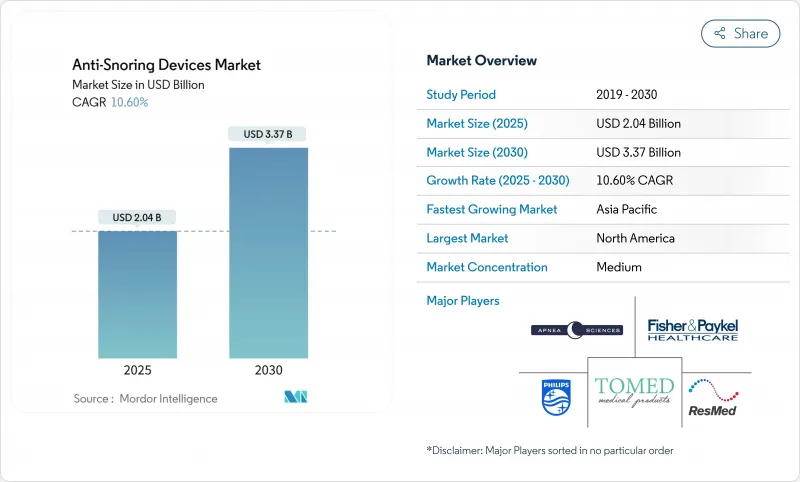
Demand growth links directly to rising obesity and aging cohorts, faster over-the-counter (OTC) approvals, and the diffusion of app-linked wearables that shorten time from diagnosis to therapy. Mandibular advancement devices (MADs) still anchor the therapeutic mix but the surge of connected positional trainers highlights consumer preference for low-profile, technology-enabled solutions. Home-care settings and online channels widen access and reduce per-patient costs, while hypoglossal nerve stimulation is redefining surgical options for Continuous Positive Airway Pressure (CPAP)-intolerant patients. CPAP adherence challenges and fragmented wellness-device regulations remain drag factors, yet overall market momentum is reinforced by corporate sleep-health programs that link therapy uptake with lower insurance premiums.
Global Anti-Snoring Devices Market Trends and Insights
Rapid Growth of Obesity & Geriatric Cohorts
Escalating obesity prevalence intersects with aging physiology to magnify obstructive sleep apnea (OSA) incidence. Each BMI unit above 30 kg/m2 accelerates apnea-hypopnea severity, while declining pharyngeal muscle tone in older adults raises airway collapsibility. U.S. modeling indicates a 26.7% increase in adult OSA prevalence between 2025 and 2030, amplifying demand for early intervention modalities that minimize downstream cardiovascular costs.
Rising Diagnosis Rates of Mild OSA via Home-Sleep Testing
Fifty-eight FDA-cleared home testing devices have lowered diagnostic barriers, with Type-3 monitors making up 84.5% of approvals. At 20-30% lower cost than lab polysomnography, home tests surface mild OSA cases that often eschew CPAP, steering clinicians toward oral appliances and positional wearables.
High Price of Custom 3-D Printed Oral Appliances
Custom devices frequently exceed USD 1,500 before impression and follow-up fees, driving total out-of-pocket cost past USD 4,000 and curbing adoption in middle-income markets. Material, printer, and post-curing expenses still prevent scale economies.
Other drivers and restraints analyzed in the detailed report include:
- Acceleration of E-Commerce DTC Sales for OTC Devices
- FDA-Cleared OTC MADs Shortening Rx-to-Therapy Cycle
- Low Long-Term Adherence to CPAP & Chin-Straps
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Mandibular advancement devices retained 39.35% 2024 anti-snoring treatment market share, while smart wearables and positional trainers chart a 14.25% CAGR, reflecting the digital pivot. Continuous Positive Airway Pressure units remain the reference for severe OSA, yet their comfort gap sustains a sizable pool for alternatives. Expiratory PAP valves and nasal dilators answer demand for minimalist airflow aids, and tongue-stabilizing tools serve anatomically specific niches.
Innovation momentum skews toward connected formats. Smart MADs layer sensors that validate nightly protrusion efficacy and transmit cloud analytics, reinforcing compliance loops. The anti-snoring treatment market size for connected oral devices is forecast to expand at 12.9% CAGR from USD 410 million in 2025, underpinned by OTC policy tailwinds. Conversely, the anti-snoring treatment industry still sees high abandonment of generic chin-straps, spotlighting design and materials upgrades as a retention lever.
Uvulopalatopharyngoplasty held 30.53% surgical share in 2024, but hypoglossal nerve stimulation's 13.85% future CAGR signals growing preference for device-guided neuromodulation. Somnoplasty and radio-frequency palatoplasty offer tissue-reduction routes with lower morbidity, while the pillar procedure fills the minimally invasive palate-stiffening niche.
Neuro-stimulation's premium price bracket (USD 30,000-40,000) restricts volume yet secures reimbursement in select markets where CPAP intolerance is documented. The anti-snoring treatment market size for nerve stimulation is forecast to reach USD 510 million by 2030, emphasizing payer-policy alignment. Viral adoption hinges on further evidence of multi-year efficacy and streamlined outpatient implantation.
The Anti-Snoring Devices Market Report is Segmented by Device Type (Mandibular Advancement Devices (MAD), and More), Surgical Intervention (Uvulopalatopharyngoplasty (UPPP), Somnoplasty, and More), End-User (Home-Care Settings, and More), Distribution Channel (Offline and Online), Technology (Connected Devices, and More), and Geography (North America, Europe, and More). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America led with 41.82% 2024 share thanks to comprehensive reimbursement, enterprise wellness initiatives, and early uptake of AI-enhanced wearables. Insurers now pilot value-based payment bundles that tie premium discounts to demonstrated adherence, reinforcing device replacement cycles. Yet CPAP drop-off rates push clinicians toward multimodal protocols, sustaining demand diversity.
Asia-Pacific posts the fastest 11.81% CAGR as demographic bulges meet rising disposable income and expanding private insurance. China alone counts 176 million OSA sufferers but only 10.25% CPAP penetration, spotlighting latent volume for lower-cost oral appliances and app-based diagnostics. Public-health campaigns now tackle entrenched cultural misperceptions; India's "Stop the Snore" initiative partners with ENT societies to normalize screening.
Europe remains a steadier, protocol-driven environment. Harmonized CE marking accelerates connected-device rollouts, while sickness-fund reimbursement stabilizes adoption curves. Middle East & Africa and South America advance from low bases; gulf states invest in specialty sleep centers, whereas Brazil's telemedicine law revisions aid remote MAD dispensing.
- Resmed
- Koninklijke Philips
- Fisher & Paykel Healthcare
- SomnoMed
- Apnea Sciences
- Whole You (Mitsui Chemicals)
- Tomed GmbH
- The Pure Sleep Company
- Signifier Medical Technologies
- ZQuiet (Sleeping Well LLC)
- Smart Nora
- Bleep LLC
- Good Sleep Co
- Rotech Healthcare
- Innovative Health
- Lear Corp (AccuMED)
- Meditas
- GlaxoSmithKline
- SnoreMeds (Pty) Ltd
- Tongue Lab
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Rapid Growth of Obesity & Geriatric Cohorts
- 4.2.2 Rising Diagnosis Rates of Mild OSA Via Home-Sleep Testing
- 4.2.3 Acceleration of E-Commerce DTC Sales For OTC Devices (Subscription Bundles)
- 4.2.4 FDA-Cleared OTC Mads Shortening Rx-To-Therapy Cycle
- 4.2.5 Smart, App-Linked Positional & Acoustic Wearables (AI Coaching)
- 4.2.6 Corporate Sleep-Health Programmes Lowering Insurance Premiums
- 4.3 Market Restraints
- 4.3.1 High Price Of Custom 3-D Printed Oral Appliances
- 4.3.2 Low Long-Term Adherence To CPAP & Chin-Straps
- 4.3.3 Fragmented Regulatory Pathways For Wellness Devices
- 4.3.4 Social Stigma In Emerging Markets Limiting Care-Seeking
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitutes
- 4.4.5 Intensity of Competitive Rivalry
5 Market Size & Growth Forecasts (Value, USD)
- 5.1 By Device Type
- 5.1.1 Mandibular Advancement Devices (MAD)
- 5.1.2 Tongue Stabilizing Devices (TSD)
- 5.1.3 Continuous Positive Airway Pressure (CPAP) Devices
- 5.1.4 Expiratory PAP (EPAP) & Nasal Dilators
- 5.1.5 Smart Wearables & Positional Trainers
- 5.1.6 Other Devices
- 5.2 By Surgical Intervention
- 5.2.1 Uvulopalatopharyngoplasty (UPPP)
- 5.2.2 Somnoplasty
- 5.2.3 Pillar Procedure
- 5.2.4 Tonsillectomy & Adenoidectomy
- 5.2.5 Radio-frequency Palatoplasty
- 5.2.6 Hypoglossal Nerve Stimulation
- 5.2.7 Laser-Assisted Uvuloplasty
- 5.2.8 Others
- 5.3 By End-user
- 5.3.1 Home-care Settings
- 5.3.2 Hospitals & Sleep Labs
- 5.3.3 Dental & ENT Clinics
- 5.4 By Distribution Channel
- 5.4.1 Offline (Hospital/Retail Pharmacies)
- 5.4.2 Online (E-commerce, DTC, Marketplaces)
- 5.5 By Technology
- 5.5.1 Connected / App-enabled Devices
- 5.5.2 Non-connected Conventional Devices
- 5.6 Geography
- 5.6.1 North America
- 5.6.1.1 United States
- 5.6.1.2 Canada
- 5.6.1.3 Mexico
- 5.6.2 Europe
- 5.6.2.1 Germany
- 5.6.2.2 United Kingdom
- 5.6.2.3 France
- 5.6.2.4 Italy
- 5.6.2.5 Spain
- 5.6.2.6 Rest of Europe
- 5.6.3 Asia-Pacific
- 5.6.3.1 China
- 5.6.3.2 Japan
- 5.6.3.3 India
- 5.6.3.4 South Korea
- 5.6.3.5 Australia
- 5.6.3.6 Rest of Asia-Pacific
- 5.6.4 Middle East and Africa
- 5.6.4.1 GCC
- 5.6.4.2 South Africa
- 5.6.4.3 Rest of Middle East and Africa
- 5.6.5 South America
- 5.6.5.1 Brazil
- 5.6.5.2 Argentina
- 5.6.5.3 Rest of South America
- 5.6.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company Profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products & Services, and Recent Developments)
- 6.3.1 ResMed
- 6.3.2 Koninklijke Philips N.V.
- 6.3.3 Fisher & Paykel Healthcare
- 6.3.4 SomnoMed
- 6.3.5 Apnea Sciences
- 6.3.6 Whole You (Mitsui Chemicals)
- 6.3.7 Tomed GmbH
- 6.3.8 The Pure Sleep Company
- 6.3.9 Signifier Medical Technologies
- 6.3.10 ZQuiet (Sleeping Well LLC)
- 6.3.11 Smart Nora
- 6.3.12 Bleep LLC
- 6.3.13 Good Sleep Co
- 6.3.14 Rotech Healthcare
- 6.3.15 Innovative Health Technologies
- 6.3.16 Lear Corp (AccuMED)
- 6.3.17 Meditas
- 6.3.18 GSK plc
- 6.3.19 SnoreMeds (Pty) Ltd
- 6.3.20 Tongue Lab
7 Market Opportunities & Future Outlook
- 7.1 White-space & Unmet-need Assessment




