PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1842432

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1842432
Ingestible Sensors - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
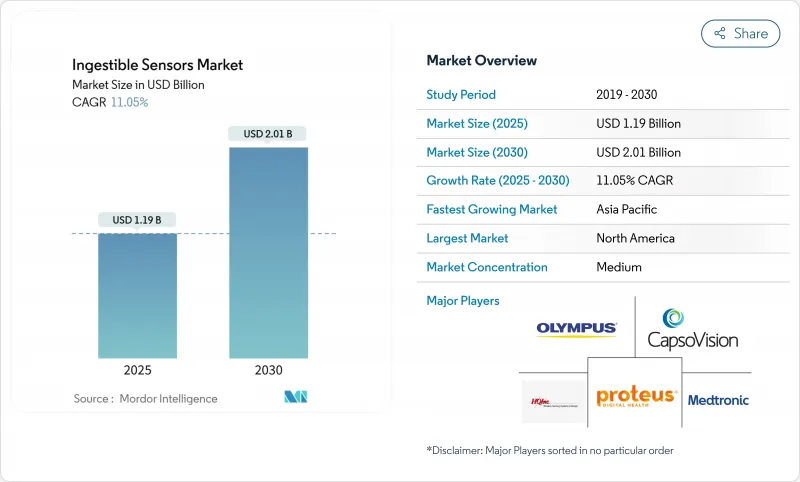
The ingestible sensors market size reached USD 1.19 billion in 2025 and is forecast to climb to USD 2.01 billion by 2030, reflecting an 11.05% CAGR.
Strong momentum stems from advances in miniaturized electronics, expanded sensing modalities, and the healthcare sector's pivot toward preventive, data-driven care. Integration of artificial intelligence with capsule-generated data is broadening real-time monitoring options for gastrointestinal disorders that once required invasive diagnostics. Regulatory clearances for digital pills are reducing market-entry barriers, while the spread of value-based reimbursement is pulling demand forward in North America and Europe. Venture funding for biosensing start-ups hit record levels in 2024, encouraging new entrants that target power efficiency and multi-parameter sensing. Nonetheless, battery capacity limits and heightened cybersecurity mandates are moderating the pace of product launches.
Global Ingestible Sensors Market Trends and Insights
Reimbursement Expansion for Digital Pills across OECD
Broader reimbursement in major OECD health systems is reinforcing predictable revenue streams for ingestible monitoring solutions. Payers link coverage to the long-term cost savings that accrue when chronic-disease patients adhere to therapy, prompting formularies to incorporate digital pills as standard options [ema.europa.eu]. Qualification of adherence sensors as valid biomarkers in European clinical trials further accelerates uptake. Hospitals now embed capsule-based adherence metrics in outcome-based contracts, anchoring demand that goes beyond early technology adopters. The resulting pull-through is expected to keep the ingestible sensors market on its double-digit growth path.
Pharma-led Push for Dose Adherence Platforms in North America
Pharmaceutical firms are embedding ingestible tags into legacy drugs to collect real-world evidence, defend pricing, and extend patent life. The FDA pathway opened by Abilify MyCite legitimized drug-device combinations, prompting others to invest heavily in similar programs. Digital ingestion data support differentiated labelling, which commands premium reimbursements and offsets the USD 100-300 billion annual burden of non-adherence. These industry moves solidify a commercial end-market that anchors early-stage sensor suppliers, sustaining the ingestible sensors market despite cyclical funding swings.
FDA Cyber-device Guidance Creating Data-Security Hurdles
Stricter 2024 cybersecurity rules obligate ingestible sensors to embed multi-layer encryption and real-time threat monitoring across their entire ecosystem [irp.nih.gov]. Meeting these standards strains power budgets and prolongs verification cycles. Smaller innovators face longer design-freeze periods and higher certification costs, tilting competitive advantage toward established firms. While the measures improve patient data integrity, they can momentarily decelerate market arrivals, dampening near-term ingestible sensors market growth projections.
Other drivers and restraints analyzed in the detailed report include:
- Miniaturized ASIC Advances Lowering Capsule Power Demand
- CE-mark Surge for In-body Telemetry Modules in EU
- Limited Capsule Battery Life Restricts Multi-Parameter Sensing.
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Temperature sensors contributed 42% of the ingestible sensors market in 2024, a position earned through validated accuracy and low power demand [sciencedirect.com]. Sports medicine, military readiness, and perioperative care rely on these capsules to avert heat stress and monitor core temperature trends. The ingestible sensors market size for temperature devices is projected to expand steadily on the back of sports league protocols that mandate continuous thermal monitoring during training blocks. Imaging capsules, despite a smaller base, are set to grow fastest at 13.8% CAGR through 2030, benefitting from miniaturized optics and expanding reimbursement for capsule endoscopy.
Image-enabled devices elevate non-invasive detection of bleeding, polyps, and Crohn's lesions, thus attracting gastroenterologists who seek to avoid sedation and endoscopic complications. Medtronic's PillCam Genius SB demonstrates how AI-assisted image sorting can reduce physician reading time while capturing tens of thousands of mucosal pictures [news.medtronic.com]. Pressure and pH modules address motility disorders and acid reflux; recent prototypes such as PressureCap integrate multiple strain gauges without inflating capsule diameter [cell.com]. Cross-modality designs that embed all three sensor types may unlock premium pricing once battery innovations alleviate power constraints.
Healthcare facilities accounted for 86% of the ingestible sensors market revenue in 2024, using capsules for medication adherence audits, bleeding localization, and inflammatory bowel disease assessment. The ingestible sensors market size tied to hospital deployment is forecast to keep growing as clinical guidelines shift endoscopy volumes toward less invasive capsule pathways. Adherence modules, cleared by the FDA for antipsychotics and antivirals, show compliance rates approaching 99%, supporting payer adoption in value-based contracts.
Elite sports teams and military organizations, though a smaller slice, form the fastest-growing customer base at a 14.2% CAGR. Thermal capsules worn by endurance athletes during events like the Olympics safeguard participants from exertional heat stroke and optimize hydration regimens. Integration with wearable heart-rate straps and cloud analytics produces a holistic training dashboard, enticing high-performance coaching staffs. Over time, consumer fitness programs may adopt simplified versions, extending the ingestible sensors market beyond professional cohorts.
Ingestible Sensors Market Segmented by Component (Sensors, Wearable Patch / Data Recorder and More), Sensor Type (Temperature Sensor, Pressure Sensor and More). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America commanded 40% of ingestible sensors market revenue in 2024, underpinned by payer reimbursement for digital pills, strong venture funding, and a supportive FDA De Novo pathway [accessdata.fda.gov]. Hospital systems deploy adherence capsules to curb costly readmissions, while pharmaceutical companies leverage real-world ingestion data to negotiate formulary placements. Regional academic centers also run early-feasibility trials that validate next-generation sensing modalities.
Asia-Pacific is forecast to chart a 14.5% CAGR from 2025 to 2030, the fastest worldwide. Japan's aging population and China's large burden of gastrointestinal disorders create a sizable addressable base. Domestic manufacturers introduce cost-optimized capsules that align with regional purchasing power, while national digital-health strategies encourage remote monitoring adoption. Government insurance in markets such as South Korea has begun considering capsule endoscopy reimbursement, further stimulating demand.
Europe retains a notable share of the ingestible sensors market, leveraging its CE-mark system, which grants earlier access to innovative telemetry capsules. Public-sector programs emphasize preventive care, aligning with non-invasive diagnostics. Increased venture funding in Germany and the Nordics supports start-ups pursuing self-powered sensors and biodegradable housings. Meanwhile, Middle East and Africa and South America together represent a small but rising opportunity; private hospitals in the Gulf Cooperation Council and Brazil are early adopters, especially for capsule endoscopy in premium care packages.
- Medtronic PLC (Given Imaging)
- Proteus Digital Health, Inc.
- CapsoVision, Inc.
- IntroMedic Co., Ltd.
- Jinshan Science and Technology
- Olympus Corporation
- HQ, Inc.
- MC10, Inc.
- etectRx, Inc.
- Otsuka Holdings Co., Ltd.
- Atmo Biosciences
- STMicroelectronics
- Philips Healthcare
- Check-Cap Ltd.
- PENTAX Medical
- RF Wireless Systems
- Karl Storz SE and Co. KG
- Boston Scientific Corporation
- CapsuleTech (a Lantronix company)
- Dassiet BioTelemetry
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET LANDSCAPE
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Reimbursement Expansion for Digital Pills across OECD
- 4.2.2 Pharma-led Push for Dose Adherence Platforms in North America
- 4.2.3 Miniaturised ASIC Advances Lowering Capsule Power Demand
- 4.2.4 CE-mark Surge for In-body Telemetry Modules in EU
- 4.2.5 Large GI Disorder Patient Pools in APAC Driving Demand
- 4.2.6 Venture Investments in Biosensing Start-ups (2023-24 record high)
- 4.3 Market Restraints
- 4.3.1 FDA Cyber-device Guidance Creating Data-Security Hurdles
- 4.3.2 Limited Capsule Battery Life Restricts Multi-parameter Sensing
- 4.3.3 Mixed Clinical Evidence on Outcome Benefits for Payors
- 4.3.4 High One-time Procedure Costs in Emerging Countries
- 4.4 Value / Supply-Chain Analysis
- 4.5 Regulatory Outlook
- 4.6 Technological Outlook
- 4.7 Patent Landscape Analysis
- 4.8 Porter's Five Forces Analysis
- 4.8.1 Threat of New Entrants
- 4.8.2 Bargaining Power of Buyers
- 4.8.3 Bargaining Power of Suppliers
- 4.8.4 Threat of Substitutes
- 4.8.5 Competitive Rivalry
5 MARKET SIZE AND GROWTH FORECASTS (VALUE)
- 5.1 By Component
- 5.1.1 Sensors
- 5.1.2 Wearable Patch / Data Recorder
- 5.1.3 Software and Analytics Platform
- 5.2 By Sensor Type
- 5.2.1 Temperature Sensor
- 5.2.2 Pressure Sensor
- 5.2.3 pH Sensor
- 5.2.4 Image Sensor
- 5.3 By Function
- 5.3.1 Imaging
- 5.3.2 Monitoring / Adherence
- 5.3.3 Drug Delivery Trigger
- 5.4 By Industry Vertical
- 5.4.1 Healthcare / Medical
- 5.4.2 Sport and Fitness
- 5.4.3 Other Verticals
- 5.5 By End-user
- 5.5.1 Hospitals and ASCs
- 5.5.2 Home Healthcare
- 5.5.3 Research Institutes
- 5.6 By Geography
- 5.6.1 North America
- 5.6.1.1 United States
- 5.6.1.2 Canada
- 5.6.1.3 Mexico
- 5.6.2 South America
- 5.6.2.1 Brazil
- 5.6.2.2 Argentina
- 5.6.2.3 Rest of South America
- 5.6.3 Europe
- 5.6.3.1 Germany
- 5.6.3.2 United Kingdom
- 5.6.3.3 France
- 5.6.3.4 Italy
- 5.6.3.5 Spain
- 5.6.3.6 Rest of Europe
- 5.6.4 Asia-Pacific
- 5.6.4.1 China
- 5.6.4.2 Japan
- 5.6.4.3 India
- 5.6.4.4 South Korea
- 5.6.4.5 Rest of Asia-Pacific
- 5.6.5 Middle East
- 5.6.5.1 Saudi Arabia
- 5.6.5.2 United Arab Emirates
- 5.6.5.3 Turkey
- 5.6.5.4 Rest of Middle East
- 5.6.6 Africa
- 5.6.6.1 South Africa
- 5.6.6.2 Nigeria
- 5.6.6.3 Rest of Africa
- 5.6.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Market Concentration
- 6.2 Strategic Moves
- 6.3 Market Share Analysis
- 6.4 Company Profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products and Services, and Recent Developments)
- 6.4.1 Medtronic PLC (Given Imaging)
- 6.4.2 Proteus Digital Health, Inc.
- 6.4.3 CapsoVision, Inc.
- 6.4.4 IntroMedic Co., Ltd.
- 6.4.5 Jinshan Science and Technology
- 6.4.6 Olympus Corporation
- 6.4.7 HQ, Inc.
- 6.4.8 MC10, Inc.
- 6.4.9 etectRx, Inc.
- 6.4.10 Otsuka Holdings Co., Ltd.
- 6.4.11 Atmo Biosciences
- 6.4.12 STMicroelectronics
- 6.4.13 Philips Healthcare
- 6.4.14 Check-Cap Ltd.
- 6.4.15 PENTAX Medical
- 6.4.16 RF Wireless Systems
- 6.4.17 Karl Storz SE and Co. KG
- 6.4.18 Boston Scientific Corporation
- 6.4.19 CapsuleTech (a Lantronix company)
- 6.4.20 Dassiet BioTelemetry
7 MARKET OPPORTUNITIES AND FUTURE OUTLOOK
- 7.1 White-space and Unmet-need Assessment




