PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1842451

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1842451
Microbial Identification - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
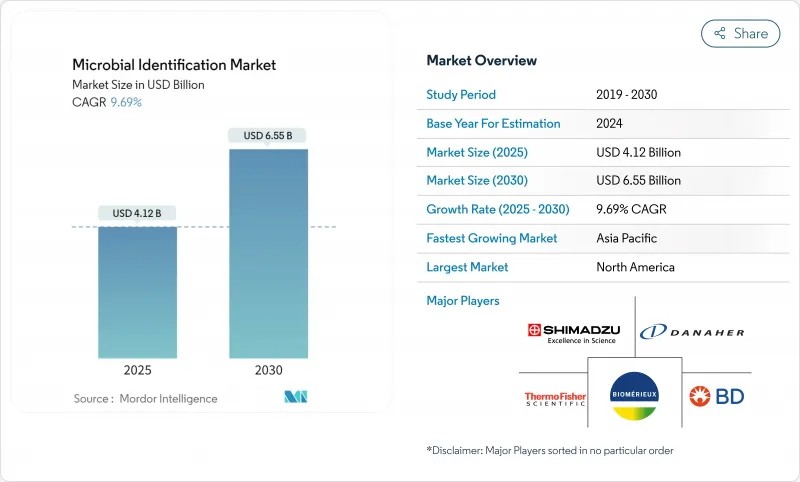
The microbial identification market was valued at USD 4.12 billion in 2025 and is forecast to reach USD 6.55 billion by 2030, advancing at a 9.69% CAGR.
The transition from culture-based assays to molecular platforms, intensified antimicrobial-resistance surveillance, and quicker turnaround expectations are the key forces sustaining momentum. Vendors are broadening technology portfolios, regulators are clarifying approval pathways, and healthcare systems are investing in real-time data integration. At the same time, staffing shortages and high capital requirements temper adoption in resource-constrained settings. Long-term growth prospects remain strong as artificial-intelligence tools extend pathogen libraries and as food-safety rules tighten across emerging economies.
Global Microbial Identification Market Trends and Insights
Rapid Adoption of MALDI-TOF MS in Routine Diagnostics
Laboratories now generate species-level identification within minutes rather than hours by using high-throughput MALDI-TOF platforms that process up to 600 samples per hour, matching the accuracy of 16S rRNA sequencing at lower reagent cost. Expanded reference databases covering more than 4,300 species enable the same instrument to support food, pharmaceutical, and clinical workflows. The United States Food and Drug Administration placed these systems in Class II with special controls in June 2025, giving manufacturers a clearer, faster clearance route while preserving safety standards .
Growth of Antimicrobial-Resistance Surveillance Programs
More than 2.8 million AMR infections occurred annually in the United States, resulting in 35,000 deaths, which prompted whole-genome sequencing adoption across surveillance networks. China's national CHINET program reported carbapenem resistance in 10% of Enterobacter isolates by 2021, highlighting convergent global pressure for rapid identification. Timely organism profiling helps pharmacists tailor effective therapy and shorten hospital stays.
High Instrument and Maintenance Costs
Capital expenditure for an advanced MALDI-TOF system can exceed USD 200,000, while service contracts add 10-15% of purchase price each year, restricting uptake in mid-tier hospitals. New Clinical Laboratory Improvement Amendments performance goals adopted in 2024 require tighter sigma metrics, which may oblige smaller labs to upgrade or replace equipment sooner than planned.
Other drivers and restraints analyzed in the detailed report include:
- Rising Food-Safety Regulations in Emerging Economies
- Integration of AI-Powered Spectral Libraries
- Shortage of Skilled Mass-Spectrometry Technicians
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Consumables generated 47.15% of 2024 revenues as labs relied on high-volume reagents and media needed for every run, giving the microbial identification market recurring cash flow resilience. Software and services, though smaller, are growing the fastest at 11.78% CAGR as laboratories upgrade to cloud laboratory-information systems that automate data movement and analytics. Next-generation "dark labs" showcasing robotics and AI illustrate how software layers mitigate staffing gaps while boosting throughput .
The shift also highlights a broader move toward subscription licensing for analytics dashboards, offering predictable margins to vendors and quicker payback for users. As quality-control regulations tighten, cloud-hosted platforms that log instrument performance and flag deviations in real time are becoming critical. This software uptake is expected to maintain double-digit growth through 2030, cementing digital processes as a core competitive differentiator across the microbial identification market.
MALDI-TOF MS retained a 57.50% revenue share in 2024 on the strength of unmatched speed-to-result, low per-test cost, and a continuously expanding organism library. The microbial identification market size for MALDI-TOF platforms is still expanding, yet growth is moderating as penetration rises in North America and Europe. PCR and real-time PCR, by contrast, will post the sharpest 12.73% CAGR through 2030 as multiplex panels and point-of-care formats reach primary-care clinics. Four separate FDA clearances for a flagship syndromic PCR analyzer in 2024 illustrate regulatory momentum.
Hybrid workflows are emerging in which laboratories first screen with MALDI-TOF, then reflex to PCR or sequencing for resistance genes, combining breadth with depth. Cross-platform data convergence is spurring new consumable and service bundles, allowing manufacturers to defend share while tapping incremental revenue from complementary molecular assays.
The Microbial Identification Market Report Segments by Products and Services (Instruments, Consumables and More), by Technology (MALDI-TOF, MSPCR & Real-Time and More ), by End User (Hospitals & Clinical Laboratories and More), by Application (Clinical Diagnostics, Pharmaceutical Manufacturing QC and More) and Geography (North America and More). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America remained the largest revenue contributor in 2024, claiming 39.56% of global spend, reflecting well-funded healthcare systems, reimbursed rapid tests, and robust AMR surveillance grants. Laboratories across the United States leverage the CDC's Antimicrobial Resistance Laboratory Network to adopt connected identification platforms that feed real-time data into national dashboards. Canada follows similar trajectories but faces greater technician shortages, delaying instrument rollouts in smaller provinces.
Asia-Pacific, forecast to rise at 11.45% CAGR, is propelled by public hospital expansion in China and India, harmonized quality standards under ASEAN initiatives, and a vibrant local biomanufacturing base. The CHINET program's multicenter datasets illustrate the region's data maturity and the resulting push for faster organism profiling to guide antibiotic formularies. Governments are also subsidizing instrument purchases for provincial disease-control centers, widening rural access.
Europe maintains moderate growth as stringent In-Vitro Diagnostic Regulation deadlines drive labs to validate platforms earlier than scheduled, ensuring steady demand for compliant kits. The United Kingdom's ESPAUR report cites a 3.5% rise in AMR burden since 2019, keeping rapid identification on policy agendas. Brexit customs changes create occasional supply chain delays, yet continental procurement frameworks largely shield end users from shortages.
The Middle East and Africa region is at an earlier adoption stage but benefits from Gulf state investment in tertiary care facilities and from donor-funded water-pathogen projects. Latin America sees rising food-safety testing volumes as Brazil and Mexico align export requirements with major trade partners, boosting uptake among agro-industry labs.
- bioMerieux
- Bruker
- Beckton Dickinson
- Thermo Fisher Scientific
- Danaher
- Shimadzu
- Charles River
- Biolog
- QIAGEN
- Merck KGaA (MilliporeSigma)
- Liofilchem Srl
- bioNote Inc.
- MIDI Labs
- Eppendorf
- Hologic
- Roche
- Siemens Healthineers
- Revvity (PerkinElmer)
- Abbott Laboratories
- Agilent Technologies
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Rapid adoption of MALDI-TOF MS in routine diagnostics
- 4.2.2 Growth of antimicrobial-resistance (AMR) surveillance programs
- 4.2.3 Rising food-safety regulations in emerging economies
- 4.2.4 Integration of AI-powered spectral libraries (under-reported)
- 4.2.5 Expansion of decentralized POCT microbial ID systems (under-reported)
- 4.3 Market Restraints
- 4.3.1 High instrument & maintenance costs
- 4.3.2 Shortage of skilled mass-spectrometry technicians
- 4.3.3 Lack of standardization for environmental isolates (under-reported)
- 4.3.4 Cyber-security risks in cloud-based ID platforms (under-reported)
- 4.4 Value/ Supply-Chain Analysis
- 4.5 Regulatory Landscape
- 4.6 Technological Outlook
- 4.7 Porter's Five Forces
- 4.7.1 Threat of New Entrants
- 4.7.2 Bargaining Power of Suppliers
- 4.7.3 Bargaining Power of Buyers
- 4.7.4 Threat of Substitutes
- 4.7.5 Competitive Rivalry
5 Market Size & Growth Forecasts (Value, USD)
- 5.1 By Product & Service
- 5.1.1 Instruments
- 5.1.2 Consumables
- 5.1.3 Software & Services
- 5.2 By Technology
- 5.2.1 MALDI-TOF MS
- 5.2.2 PCR & Real-time PCR
- 5.2.3 Sequencing (NGS, Sanger)
- 5.2.4 Others (Biochemical, Microscopy, etc.)
- 5.3 By End-User
- 5.3.1 Hospitals & Clinical Laboratories
- 5.3.2 Pharmaceutical & Biotechnology Companies
- 5.3.3 Food & Beverage Testing Labs
- 5.3.4 Environmental & Industrial Labs
- 5.4 By Application
- 5.4.1 Clinical Diagnostics
- 5.4.2 Pharmaceutical Manufacturing QC
- 5.4.3 Food Safety & Quality
- 5.4.4 Environmental Monitoring
- 5.5 By Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.2 Europe
- 5.5.2.1 Germany
- 5.5.2.2 United Kingdom
- 5.5.2.3 France
- 5.5.2.4 Italy
- 5.5.2.5 Spain
- 5.5.2.6 Rest of Europe
- 5.5.3 Asia-Pacific
- 5.5.3.1 China
- 5.5.3.2 India
- 5.5.3.3 Japan
- 5.5.3.4 South Korea
- 5.5.3.5 Australia
- 5.5.3.6 Rest of Asia-Pacific
- 5.5.4 South America
- 5.5.4.1 Brazil
- 5.5.4.2 Argentina
- 5.5.4.3 Rest of South America
- 5.5.5 Middle East and Africa
- 5.5.5.1 GCC
- 5.5.5.2 South Africa
- 5.5.5.3 Rest of Middle East and Africa
- 5.5.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company Profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share, Products & Services, Recent Developments)
- 6.3.1 bioMerieux SA
- 6.3.2 Bruker Corporation
- 6.3.3 Becton, Dickinson and Company
- 6.3.4 Thermo Fisher Scientific Inc.
- 6.3.5 Danaher Corporation (Beckman Coulter)
- 6.3.6 Shimadzu Corporation
- 6.3.7 Charles River Laboratories
- 6.3.8 Biolog Inc.
- 6.3.9 Qiagen N.V.
- 6.3.10 Merck KGaA (MilliporeSigma)
- 6.3.11 Liofilchem Srl
- 6.3.12 bioNote Inc.
- 6.3.13 MIDI Labs
- 6.3.14 Eppendorf AG
- 6.3.15 Hologic Inc.
- 6.3.16 Roche Diagnostics
- 6.3.17 Siemens Healthineers
- 6.3.18 Revvity (PerkinElmer)
- 6.3.19 Abbott Laboratories
- 6.3.20 Agilent Technologies
7 Market Opportunities & Future Outlook
- 7.1 White-space & Unmet-Need Assessment




