PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1842462

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1842462
Surgical Drains - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
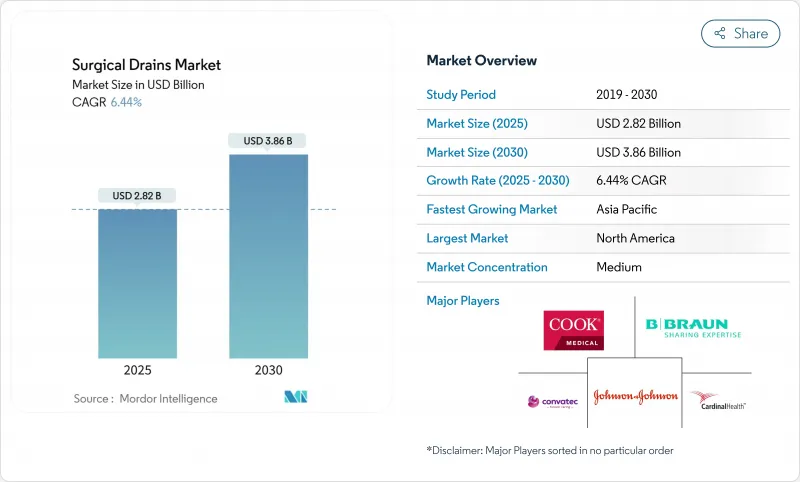
The surgical drains market stands at USD 2.82 billion in 2025 and is projected to expand to USD 3.86 billion by 2030, advancing at a 6.44% CAGR.
Growing case complexity, rapid digital adoption, and patient-centric care protocols are shaping demand patterns. Hospitals invest in drainage systems that link seamlessly with electronic health records, while ambulatory sites seek devices that support same-day discharge. Advanced infection-control requirements push manufacturers toward antimicrobial materials and closed-system designs. Simultaneously, Asia-Pacific procedure volumes surge as governments channel capital toward new surgical centers, attracting global suppliers and stimulating local production.
Global Surgical Drains Market Trends and Insights
Growing Volume of Complex Surgeries
Higher case complexity boosts demand for devices that manage multifaceted fluid dynamics. Medicare reported 3.3 million beneficiaries in U.S. ambulatory centers during 2022, underscoring procedure growth that elevates drainage requirements. Enhanced recovery pathways still rely on drains for cardiovascular, orthopedic, and neurosurgical interventions that involve sizable fluid shifts. Aging populations ensure continued growth in joint replacements, and minimally invasive approaches require slimline drains that accommodate small incisions. These forces sustain premium system sales within the surgical drains market.
Rapid Adoption of Digital / Smart Drainage Systems
Real-time fluid analytics shorten hospital stays and cut manual checks. Clinical evaluations of digital chest drains confirmed faster tube removal and reduced length of stay.IoT-enabled devices transmit output metrics to nursing dashboards, easing workloads and enhancing early-warning capability. Hospitals justify upfront costs through downstream efficiency gains. In parallel, ambulatory centers deploy compact smart units that support home recovery and remote oversight.
Adverse Events & Litigation Over Retained Drains
Retained fragments can trigger gait disturbances and long-term disability, as documented after total hip arthroplasty. Institutions facing liability tighten protocols, eliminating drains where evidence shows negligible benefit. This caution directly moderates device volumes, especially in high-litigation regions.
Other drivers and restraints analyzed in the detailed report include:
- Higher Infection-Control Standards in Ambulatory Settings
- Shift Toward Day-Care Surgery & ERAS Protocols
- Accelerating Pivot to Minimally-Invasive & Drain-Less Techniques
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Surgical drainage systems generated 58.76% of revenue in 2024 and are forecast to outpace accessories with a 9.24% CAGR. Demand centers on connected platforms that log output, pressure, and alarm data inside the hospital information backbone. Such performance underpins premium pricing in the surgical drains market. Accessories, while steady, feel commoditization as integrated kits bundle tubing, connectors, and dressings.
Replacement frequency sustains accessory revenue, yet growth trails system upgrades. Novel silicone fixation dressings improve comfort and reduce re-taping, hinting at incremental gains. System makers blur category lines by embedding securement features within primary devices, consolidating procurement and simplifying inventory.
Active units held 59.45% share in 2024 and should grow 8.23% annually through 2030. Precision suction and flow regulation fit critical thoracic and cardiac cases that demand tight pressure control. The Interi System, for instance, lowered seroma rates from 22.9% to 4.1% in breast reconstruction. Passive drains thrive in price-sensitive settings; however, their limited monitoring restricts adoption in high-acuity wards.
Emerging reimbursement models reward outcome tracking, reinforcing the appeal of active drains that document performance metrics. Passive silicone variants remain indispensable for low-resource environments, helping preserve surgical access where budgets constrain upgrades.
The Surgical Drains Market Report is Segmented by Product (Surgical Drainage Systems [Open Surgical Drainage Systems and More and Accessories), Type (Active Drains and Passive Drains), Application (Thoracic and Cardiovascular Surgery, Neurosurgery, and More) End User (Hospitals, and Ambulatory Surgical Centers & Clinics) and Geography (North America, Europe, and More). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America retained 36.71% of global revenue in 2024 owing to high procedure counts and early digital uptake. Medicare outlays of USD 6.1 billion for ambulatory surgeries in 2022 attest to robust demand for advanced postoperative care devices. Hospitals emphasize outcome documentation, incentivizing connected drains that feed data to quality dashboards. Supply disruptions highlight vendors able to guarantee on-time delivery.
Europe delivers stable gains anchored in aging demographics and stringent infection prevention rules. ERAS adoption reshapes purchasing by valuing devices that enable early mobilization. National health systems in Western Europe weigh total cost of ownership heavily, rewarding suppliers who quantify reductions in length of stay and complication rates.
Asia-Pacific is the surgical drains market growth hotspot with an 8.19% CAGR forecast. Governments funnel capital into new surgical suites and encourage domestic production through tax holidays. While China's tender rules favor local brands, quality gaps in advanced smart systems leave space for collaborations with multinational firms. Medical tourism hubs such as Thailand and India also fuel device imports, particularly for complex cardiovascular and oncology procedures.
- B. Braun
- Cardinal Health
- ConvaTec PLC
- Cook Group
- Degania Silicone
- Johnson & Johnson
- Hollister
- Poly Medicure
- Romsons
- Zimmer Biomet
- Medtronic
- Teleflex
- Redax
- Medela
- Argon Medical Devices
- Canack Technology
- Pfm Medical
- Hospitech
- Stryker
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Growing Volume Of Complex Surgeries
- 4.2.2 Rapid Adoption Of Digital / Smart Drainage Systems
- 4.2.3 Higher Infection-Control Standards In Ambulatory Settings
- 4.2.4 Shift Toward Day-Care Surgery & Eras Protocols
- 4.2.5 Value-Based Procurement In High-Income Markets
- 4.2.6 Local Production Incentives In Few Countries
- 4.3 Market Restraints
- 4.3.1 Adverse Events & Litigation Over Retained Drains
- 4.3.2 Accelerating Pivot To Minimally-Invasive & Drain-Less Techniques
- 4.3.3 Supply-Chain Shortages In Medical-Grade Silicone
- 4.3.4 Hospitals Delaying Cap-Ex Amid Reimbursement Pressure
- 4.4 Value / Supply-Chain Analysis
- 4.5 Regulatory Landscape
- 4.6 Technology Outlook
- 4.7 Porter's Five Forces Analysis
- 4.7.1 Bargaining Power of Suppliers
- 4.7.2 Bargaining Power of Buyers
- 4.7.3 Threat of New Entrants
- 4.7.4 Threat of Substitutes
- 4.7.5 Intensity of Competitive Rivalry
5 Market Size and Growth Forecasts (Value-USD)
- 5.1 By Product
- 5.1.1 Surgical Drainage Systems
- 5.1.1.1 Open Surgical Drainage Systems
- 5.1.1.2 Closed Surgical Drainage Systems
- 5.1.2 Accessories
- 5.1.1 Surgical Drainage Systems
- 5.2 By Type
- 5.2.1 Active Drains
- 5.2.2 Passive Drains
- 5.3 By Application
- 5.3.1 Thoracic & Cardiovascular Surgery
- 5.3.2 Neurosurgery
- 5.3.3 Abdominal Surgery
- 5.3.4 Orthopedics
- 5.3.5 Others
- 5.4 By End User
- 5.4.1 Hospitals
- 5.4.2 Ambulatory Surgical Centers & Clinics
- 5.5 By Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.2 Europe
- 5.5.2.1 Germany
- 5.5.2.2 United Kingdom
- 5.5.2.3 France
- 5.5.2.4 Italy
- 5.5.2.5 Spain
- 5.5.2.6 Rest of Europe
- 5.5.3 Asia-Pacific
- 5.5.3.1 China
- 5.5.3.2 Japan
- 5.5.3.3 India
- 5.5.3.4 Australia
- 5.5.3.5 South Korea
- 5.5.3.6 Rest of Asia-Pacific
- 5.5.4 Middle East and Africa
- 5.5.4.1 GCC
- 5.5.4.2 South Africa
- 5.5.4.3 Rest of Middle East and Africa
- 5.5.5 South America
- 5.5.5.1 Brazil
- 5.5.5.2 Argentina
- 5.5.5.3 Rest of South America
- 5.5.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products and Services, and Recent Developments)
- 6.3.1 B. Braun
- 6.3.2 Cardinal Health
- 6.3.3 ConvaTec PLC
- 6.3.4 Cook Medical
- 6.3.5 Degania Silicone
- 6.3.6 Johnson & Johnson
- 6.3.7 Hollister Incorporated
- 6.3.8 Poly Medicure
- 6.3.9 Romsons
- 6.3.10 Zimmer Biomet
- 6.3.11 Medtronic
- 6.3.12 Teleflex
- 6.3.13 Redax
- 6.3.14 Medela
- 6.3.15 Argon Medical Devices
- 6.3.16 Canack Technology
- 6.3.17 Pfm Medical
- 6.3.18 Hospitech
- 6.3.19 Stryker
7 Market Opportunities and Future Outlook
- 7.1 White-Space and Unmet-Need Assessment




