PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1842588

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1842588
Bronchitis Treatment - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
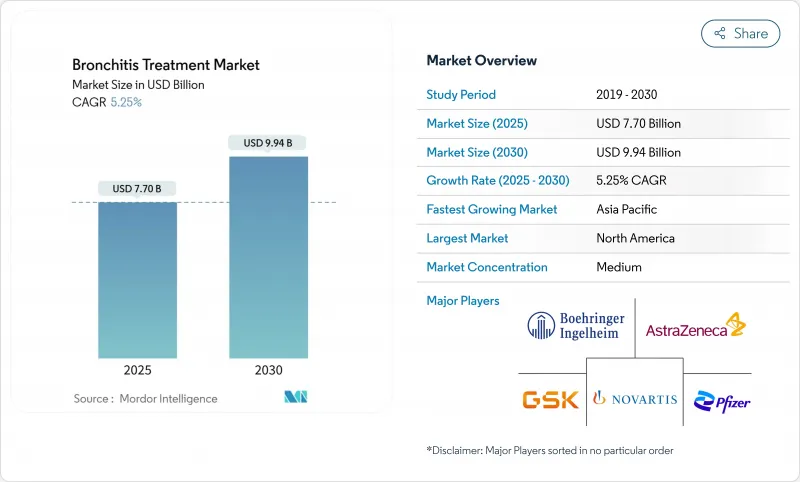
The bronchitis treatment market stood at USD 7.70 billion in 2025 and is on course to reach USD 9.94 billion by 2030, advancing at a 5.25% CAGR over the forecast period.
Increasing life expectancy, sustained urban air-pollution exposure, and the wider adoption of long-acting inhaled combination therapies are reshaping commercial opportunity. A steady rise in chronic obstructive pulmonary disease (COPD) prevalence, especially among aging populations, keeps patient volumes high, while megacity nano-particle concentrations continue to elevate symptomatic caseloads. Digital health integration further broadens treatment access through home-monitoring platforms that improve adherence and reduce costly exacerbations. Supply-side pressure persists, however, as antibiotic active-ingredient sourcing remains concentrated in a few Asian hubs, leading firms to diversify manufacturing footprints to reinforce resilience.
Global Bronchitis Treatment Market Trends and Insights
Growing Geriatric Population With Higher Bronchitis Incidence
People aged 65 and above now account for an unprecedented share of the global population, and molecular hallmarks of lung aging-oxidative stress and cellular senescence-reduce mucociliary clearance and impair immune function. As a result, elderly patients present with more frequent bronchitis episodes requiring intensive pharmacologic support. Health systems are responding by rolling out age-specific care pathways, geriatric pulmonary clinics, and home-based monitoring to avoid hospital readmissions. Drug developers are tailoring dosing regimens and delivery devices to accommodate declining inspiratory flow, further stimulating the bronchitis treatment market.
Escalating COPD Prevalence Worldwide
Global COPD prevalence continues to climb, especially in middle-income economies where tobacco exposure and indoor biomass fuel use remain common. Because chronic bronchitis is a core COPD phenotype, escalating COPD caseloads translate directly into sustained demand for long-acting bronchodilators and dual anti-inflammatory therapies. The 2024 FDA approval of ensifentrine, the first dual PDE3/4 inhibitor for maintenance therapy in over two decades, signals industry commitment to novel mechanisms that reduce exacerbations and enhance quality of life.
Stringent Drug-Approval Timelines & Costs
Securing a new respiratory therapy can require close to USD 1 billion in R&D outlay and nearly eight years of clinical development, while a single FDA application with clinical data now commands a USD 4.31 million fee. Such economics discourage smaller innovators and slow the refresh rate of first-in-class molecules, tempering the pace at which groundbreaking options reach the bronchitis treatment market.
Other drivers and restraints analyzed in the detailed report include:
- Rising Air-Pollution Nano-Particle Exposure In Megacities
- Expanding Tele-Pulmonology Platforms Improving Therapy Adherence
- Volatile API Supply Chains For Macrolides & Quinolones
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Antibiotics held 36.55% of bronchitis treatment market share in 2024, reflecting their entrenched role for bacterial exacerbations. The bronchitis treatment market size for antibiotics reached USD 2.81 billion and expanded modestly as stewardship programs temper unnecessary use. Concurrently, bronchodilators registered a 7.25% CAGR, buoyed by the launch of dual-mechanism inhalers and triple fixed-dose combinations. The bronchodilator segment of bronchitis treatment market size is forecast to surpass USD 2.3 billion by 2030 as payers increasingly recognize their exacerbation-prevention value.
Regulatory tailwinds favor long-acting formulations: the June 2024 FDA approval of ensifentrine reinvigorated R&D pipelines. Antibiotic innovators counter by reformulating macrolides for once-daily dosing and rapid-onset parenteral options. In parallel, herbal alternatives such as ivy-leaf extract achieve guideline endorsements for acute bronchitis relief, reflecting growing consumer interest in antibiotic-sparing therapies.
Acute bronchitis contributed 59.53% to 2024 revenue yet grows slowly as viral etiology awareness restricts antibiotic prescribing. Chronic bronchitis, however, is advancing at a 9.35% CAGR and will close the gap by the end of the decade, underpinned by the aging population and mounting COPD burden. Payers increasingly reimburse maintenance therapies that curb hospitalizations, bolstering the chronic segment's share of bronchitis treatment market size.
Guideline updates now stress early introduction of inhaled anti-inflammatory combinations for chronic cases, a shift mirrored by rising uptake of smart inhalers capable of logging real-world adherence and inspiratory flow metrics. Acute bronchitis care continues migrating toward symptomatic relief, with rapid diagnostics supporting delayed antibiotic scripts that meet antimicrobial stewardship targets.
The Bronchitis Treatment Market Report is Segmented by Class of Drugs (Antibiotics, Anti-Inflammatory Drugs, Bronchodilators, and More), Disease Type (Acute Bronchitis and Chronic Bronchitis), Distribution Channel (Hospital Pharmacies, Retail Pharmacies, and More), Route of Administration (Oral, Inhalation, and Parenteral), and Geography (North America, Europe, and More). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America controlled 35.82% of 2024 revenue thanks to cutting-edge drug availability, comprehensive reimbursement frameworks, and high telehealth penetration. Recent FDA approvals of biologics such as mepolizumab for eosinophilic COPD have widened the therapeutic arsenal, supporting premium pricing in the United States. Canada's single-payer system negotiates lower list prices yet drives volume through national COPD screening programs, while Mexico benefits from cross-border generic importation that reduces out-of-pocket costs.
Asia-Pacific is expanding fastest at a 10.52% CAGR as governments boost healthcare outlays and encourage local production of complex inhaled formulations. China's Healthy China 2030 agenda increases diagnosis rates, and India's Ayushman Bharat scheme enlarges insurance coverage, collectively lifting treatment uptake. Singapore's 2024 clearance of Trelegy Ellipta underscores the region's growing role as a launchpad for inhaled triple therapies. However, regulatory heterogeneity across ASEAN markets necessitates tailored filing pathways, elongating time-to-launch.
Europe maintains steady growth, propelled by universal coverage and robust antimicrobial stewardship that nudges prescribers toward non-antibiotic options. The EU's heightened focus on clean-air directives indirectly supports preventive treatment demand as cities struggle to meet PM2.5 targets. Eastern European states observe rapid uptake of generic bronchodilators, while Western markets embrace biologic add-ons for severe phenotypes. Elsewhere, South America and the Middle East & Africa offer long-term upside but grapple with volatile currency environments and patchy insurance coverage, prompting multinational firms to partner with local distributors for wider reach.
- AstraZeneca
- Novartis
- GlaxoSmithKline
- Boehringer Ingelheim
- Pfizer
- Sanofi
- Dr. Reddy's Laboratories
- Lupin
- Zydus Group
- Melinta Therapeutics
- Merck
- Roche
- Teva Pharmaceutical Industries
- Kenvue Inc.
- Cipla
- Insmed
- CSL Behring
- Chiesi Farmaceutici
- Zambon S.p.A.
- Armata Pharmaceuticals
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Growing Geriatric Population With Higher Bronchitis Incidence
- 4.2.2 Escalating COPD Prevalence Worldwide
- 4.2.3 Rising Air-Pollution Nano-Particle Exposure In Megacities
- 4.2.4 Expanding Tele-Pulmonology Platforms Improving Therapy Adherence
- 4.2.5 Government Push For AMR-Conscious Antibiotic Stewardship
- 4.3 Market Restraints
- 4.3.1 Stringent Drug-Approval Timelines & Costs
- 4.3.2 Volatile API Supply Chains For Macrolides & Quinolones
- 4.3.3 Growing Consumer Preference For Antibiotic-Free Herbal Remedies
- 4.4 Porter's Five Forces
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitutes
- 4.4.5 Intensity of Competitive Rivalry
5 Market Size & Growth Forecasts (Value, USD)
- 5.1 By Class of Drugs
- 5.1.1 Antibiotics
- 5.1.2 Anti-inflammatory Drugs
- 5.1.3 Bronchodilators
- 5.1.4 Mucolytics & Expectorants
- 5.1.5 Other Drugs
- 5.2 By Disease Type
- 5.2.1 Acute Bronchitis
- 5.2.2 Chronic Bronchitis
- 5.3 By Distribution Channel
- 5.3.1 Hospital Pharmacies
- 5.3.2 Retail Pharmacies
- 5.3.3 Online & Mail-order Pharmacies
- 5.3.4 Other End Users
- 5.4 By Route of Administration
- 5.4.1 Oral
- 5.4.2 Inhalation
- 5.4.3 Parenteral
- 5.5 Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.2 Europe
- 5.5.2.1 Germany
- 5.5.2.2 United Kingdom
- 5.5.2.3 France
- 5.5.2.4 Italy
- 5.5.2.5 Spain
- 5.5.2.6 Rest of Europe
- 5.5.3 Asia-Pacific
- 5.5.3.1 China
- 5.5.3.2 Japan
- 5.5.3.3 India
- 5.5.3.4 South Korea
- 5.5.3.5 Australia
- 5.5.3.6 Rest of Asia-Pacific
- 5.5.4 Middle East and Africa
- 5.5.4.1 GCC
- 5.5.4.2 South Africa
- 5.5.4.3 Rest of Middle East and Africa
- 5.5.5 South America
- 5.5.5.1 Brazil
- 5.5.5.2 Argentina
- 5.5.5.3 Rest of South America
- 5.5.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company Profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products & Services, and Recent Developments)
- 6.3.1 AstraZeneca PLC
- 6.3.2 Novartis AG
- 6.3.3 GSK PLC
- 6.3.4 Boehringer Ingelheim International GmbH
- 6.3.5 Pfizer Inc.
- 6.3.6 Sanofi SA
- 6.3.7 Dr. Reddy's Laboratories Ltd
- 6.3.8 Lupin Limited
- 6.3.9 Cadila Healthcare Limited
- 6.3.10 Melinta Therapeutics
- 6.3.11 Merck & Co., Inc.
- 6.3.12 Roche Holding AG
- 6.3.13 Teva Pharmaceutical Industries Ltd.
- 6.3.14 Kenvue Inc.
- 6.3.15 Cipla Ltd.
- 6.3.16 Insmed Incorporated
- 6.3.17 CSL Behring
- 6.3.18 Chiesi Farmaceutici S.p.A.
- 6.3.19 Zambon S.p.A.
- 6.3.20 Armata Pharmaceuticals
7 Market Opportunities & Future Outlook
- 7.1 White-space & Unmet-need Assessment




