PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1842591

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1842591
Aseptic Sampling - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
The aseptic sampling market stands at USD 1.26 billion in 2025 and is on track to reach USD 2.24 billion by 2030, advancing at a 12.22% CAGR.
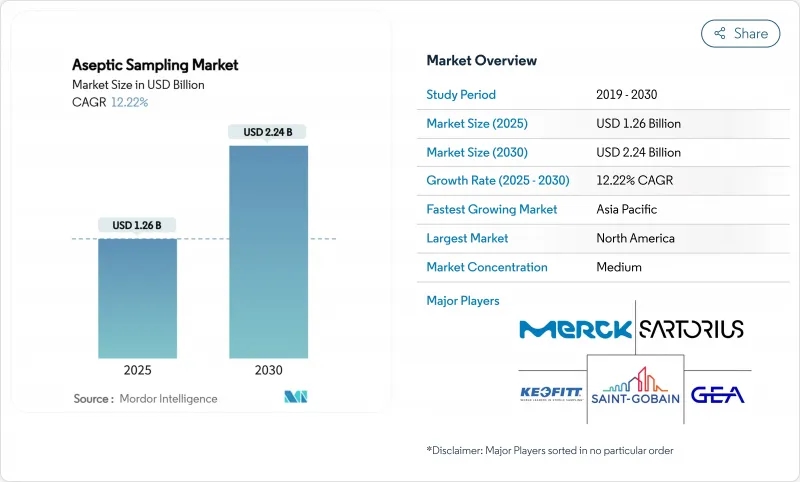
Rapid investment in contamination-free bioprocessing, tighter sterility regulations, and wider adoption of single-use assemblies underpin this expansion. Pharmaceutical producers see automated devices as a reliable guardrail against human error, while growing cell and gene therapy pipelines force sterility controls earlier in development. Digital process analytical technology (PAT) now pairs with sampling hardware to deliver real-time quality data that protects multimillion-dollar biologic batches. Regionally, North American manufacturers defend leadership through mature infrastructure and FDA oversight, yet Asia-Pacific facilities add capacity faster on the back of state incentives and lower operating costs. Competition intensifies as integrated solution providers couple hardware, analytics, and data management into unified platforms that shorten validation timelines.
Global Aseptic Sampling Market Trends and Insights
Stringent Government Regulations For Sterility Assurance
Global regulators now require tighter sampling frequency and traceability in aseptic production. The revised FDA guidance extends to advanced therapy medicinal products and mandates routine environmental monitoring with documented validation. Equivalent EU GMP Annex 1 revisions align expectations across regions, prompting manufacturers to replace paper logs with electronic audit trails and automated devices that record every intervention. This pressure accelerates upgrades in legacy plants and prescribes closed, single-use pathways in greenfield sites to minimize contamination risks.
Rapid Scale-Up Of Cell & Gene Therapy Pipelines
Commercialization of autologous and allogeneic therapies exposes sterility weak points, as each patient batch carries zero tolerance for cross-contamination. Producers therefore specify automated, closed sampling that secures chain-of-custody documentation and supports diverse viral vectors and cell types. As approvals approach 3,000 therapies by 2030, capacity build-outs demand modular skids that drop into multiproduct suites without lengthy validation cycles.
Leachables & Extractables Risk In Polymeric Assemblies
Disposable manifolds can release organic acids, plasticizers, or trace metals that destabilize sensitive biologics, requiring exhaustive chemical profiling. Firms often run multi-week extractables studies at several temperatures and solvents, adding cost and delaying product launch schedules. The absence of harmonized global test standards also multiplies analytical workloads.
Other drivers and restraints analyzed in the detailed report include:
- Shift Toward Closed-Loop, Single-Use Bioprocessing
- In-Line, At-Line PAT Adoption Improving Batch Yields
- High CAPEX Of Automated Aseptic Sampling Skids
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Manual systems commanded a 72.35% share of the aseptic sampling market in 2024. Their low capital outlay and proven compliance records maintain widespread usage, especially in legacy plants where infrastructure changes invite downtime. However, automated modules exhibit the fastest 18.25% CAGR as producers target lower operator exposure and stronger data integrity. Automated skids integrate with manufacturing execution systems to log every grab and immediately archive results for audit review. That capability relieves documentation fatigue and elevates confidence during FDA inspections. Rising batch values in cellular therapies sharpen demand for solutions that remove human interventions entirely, reinforcing the long-term tilt toward automation in the aseptic sampling market.
Manual kits still occupy niches such as early R&D or low-volume biologics where budget trumps throughput. Vendors now position hybrid platforms that accept manual triggers yet automate sterilization between uses. This bridge strategy helps price-sensitive buyers migrate gradually without scrapping existing protocols. Over the forecast window, wider harmonization of electronic records standards is set to catalyze a decisive inflection toward automated devices as the default for commercial production lines within the aseptic sampling market.
On-line instruments represented 46.53% of global revenue in 2024 due to their real-time feedback. They continuously pull micro-aliquots under closed conditions, enabling immediate pH or nutrient adjustments. At-line devices, posting the brisk 13.85% CAGR, attract operators who want frequent analytics without the engineering complexity of fully integrated on-line loops. At-line probes station adjacent to the vessel, keep tubing lengths short, and permit rapid sensor swaps. This reduces risk of clogging and simplifies calibrations.
Off-line grabs persist for advanced analytics such as viral clearance assays that cannot be miniaturized. Yet every off-line transfer involves open handling, elongates turnaround, and risks deviations. As PAT guidelines and real-time release testing mature, at-line units will likely siphon incremental share from off-line workflows. Standardized mechanical interfaces and disposable flow paths now make retrofits easier, bolstering adoption across mid-tier plants in the aseptic sampling market.
The Aseptic Sampling Market Report is Segmented by Type of Sampling (Manual Aseptic Sampling and Automated Aseptic Sampling), Sampling Technique (On-Line Sampling and More), Application (Upstream Process and Downstream Process), End-User (Biotechnology and Pharmaceutical Manufacturers and More), Component Material (Single-Use Assemblies and Reusable Systems), and Geography. The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America captured 41.82% revenue in 2024 and defends its lead through deep biopharmaceutical pipelines, benchmarking FDA guidance, and a density of CDMOs. The United States hosts most commercial cell therapy facilities and invests heavily in PAT. Canada builds biosimilar capacity under targeted federal grants, while Mexico's cost-effective labor spurs generic drug output. Demand rises further due to venture capital backing first-in-class biologics that demand heightened sterility.
Asia-Pacific records the strongest 13.61% CAGR as governments subsidize capacity and enforce quality uplift. South Korea's SK Pharmteco placed USD 260 million into peptide synthesis lines equipped with closed samplers. China's localization policies call for domestic supply security, fueling new biologics parks that standardize single-use sampling from the outset. India remains a powerhouse for active pharmaceutical ingredients, where cost-sensitive plants mix manual and disposable kits. Collectively, these programs push the aseptic sampling market deeper into the region and establish local manufacturing bases for global vendors.
Europe remains stable, anchored by Germany's engineering clusters and France's biologics expansions. Post-Brexit United Kingdom facilities align with updated Annex 1, driving retrofits of automated sampling loggers. Sustainability regulations pressure producers to explore hybrid metal-plastic manifolds and to document lifecycle impacts. Italian and Spanish vaccine manufacturers similarly upgrade equipment to secure pandemic preparedness grants. These investments deliver steady, if less dramatic, growth that keeps the region an innovation hub for the aseptic sampling market.
- Merck
- Sartorius
- Thermo Fisher Scientific
- Danaher Corp (Pall & Cytiva)
- Lonza Group
- Keofitt
- Saint-Gobain Life Sciences
- GEA Group
- Gemu Group
- Qualitru Sampling Systems
- W. L. Gore & Associates
- Avantor (VWR)
- Repligen Corp
- Solventum Corporation
- Parker Hannifin (domnick hunter)
- Colder Products Company (CPC)
- Mettler-Toledo
- PendoTECH
- Bbi-Biotech GmbH
- Advanced Microdevices Pvt Ltd
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Stringent Government Regulations For Sterility Assurance
- 4.2.2 Rapid Scale-Up Of Cell & Gene Therapy Pipelines
- 4.2.3 Shift Toward Closed-Loop, Single-Use Bioprocessing
- 4.2.4 In-Line, At-Line PAT Adoption Improving Batch Yields
- 4.2.5 AI-Driven Contamination Prediction Platforms
- 4.3 Market Restraints
- 4.3.1 Leachables & Extractables Risk In Polymeric Assemblies
- 4.3.2 High CAPEX Of Automated Aseptic Sampling Skids
- 4.3.3 Complex Validation For Multi-Use Connectors
- 4.4 Porter's Five Forces
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitutes
- 4.4.5 Intensity of Competitive Rivalry
5 Market Size & Growth Forecasts (Value, USD)
- 5.1 By Type of Sampling
- 5.1.1 Manual Aseptic Sampling
- 5.1.1.1 Bags
- 5.1.1.2 Bottles
- 5.1.1.3 Other Containers
- 5.1.2 Automated Aseptic Sampling
- 5.1.1 Manual Aseptic Sampling
- 5.2 By Sampling Technique
- 5.2.1 On-line Sampling
- 5.2.2 At-line Sampling
- 5.2.3 Off-line Sampling
- 5.3 By Application
- 5.3.1 Upstream Process
- 5.3.2 Downstream Process
- 5.4 By End-User
- 5.4.1 Biotechnology & Pharmaceutical Manufacturers
- 5.4.2 Contract Research & Manufacturing Organizations
- 5.4.3 Academic & Research Institutes
- 5.5 By Component Material
- 5.5.1 Single-Use Assemblies
- 5.5.2 Reusable (Stainless-Steel-Based) Systems
- 5.6 Geography
- 5.6.1 North America
- 5.6.1.1 United States
- 5.6.1.2 Canada
- 5.6.1.3 Mexico
- 5.6.2 Europe
- 5.6.2.1 Germany
- 5.6.2.2 United Kingdom
- 5.6.2.3 France
- 5.6.2.4 Italy
- 5.6.2.5 Spain
- 5.6.2.6 Rest of Europe
- 5.6.3 Asia-Pacific
- 5.6.3.1 China
- 5.6.3.2 Japan
- 5.6.3.3 India
- 5.6.3.4 South Korea
- 5.6.3.5 Australia
- 5.6.3.6 Rest of Asia-Pacific
- 5.6.4 Middle East and Africa
- 5.6.4.1 GCC
- 5.6.4.2 South Africa
- 5.6.4.3 Rest of Middle East and Africa
- 5.6.5 South America
- 5.6.5.1 Brazil
- 5.6.5.2 Argentina
- 5.6.5.3 Rest of South America
- 5.6.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company Profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products & Services, and Recent Developments)
- 6.3.1 Merck KGaA
- 6.3.2 Sartorius AG
- 6.3.3 Thermo Fisher Scientific
- 6.3.4 Danaher Corp (Pall & Cytiva)
- 6.3.5 Lonza Group
- 6.3.6 Keofitt A/S
- 6.3.7 Saint-Gobain Life Sciences
- 6.3.8 GEA Group
- 6.3.9 Gemu Group
- 6.3.10 Qualitru Sampling Systems
- 6.3.11 W. L. Gore & Associates
- 6.3.12 Avantor (VWR)
- 6.3.13 Repligen Corp
- 6.3.14 Solventum Corporation
- 6.3.15 Parker Hannifin (domnick hunter)
- 6.3.16 Colder Products Company (CPC)
- 6.3.17 Mettler-Toledo
- 6.3.18 PendoTECH
- 6.3.19 Bbi-Biotech GmbH
- 6.3.20 Advanced Microdevices Pvt Ltd
7 Market Opportunities & Future Outlook
- 7.1 White-space & Unmet-need Assessment




