PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1842611

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1842611
Kaposi Sarcoma - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
The Kaposi's Sarcoma treatment market stands at USD 144.57 billion in 2025 and is forecast to reach USD 176.71 billion by 2030, advancing at a 4.1% CAGR.
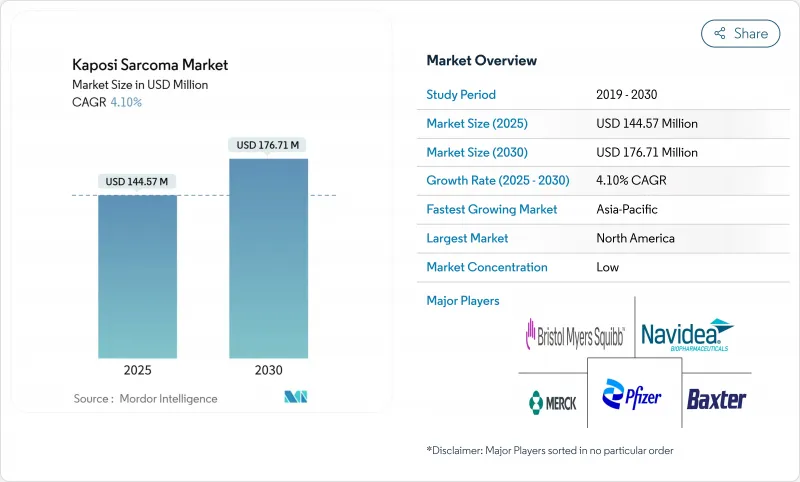
Demand is guided more by precision-medicine advances than by sheer patient volume, so growth remains steady rather than explosive. Rising incidence among immuno-compromised populations, wider use of pegylated liposomal anthracyclines, and pipeline immune-checkpoint inhibitors together reinforce this upward trajectory, while cold-chain gaps and reimbursement headwinds temper the pace. Consolidation among leading oncology businesses is intensifying, with big-ticket acquisitions exceeding USD 10 billion in 2024 alone. Simultaneously, cell-based gene therapies and AI-driven pathology platforms are shortening diagnostic lead-times, unlocking earlier intervention opportunities that further expand the addressable patient pool.
Global Kaposi Sarcoma Market Trends and Insights
Rising Incidence Among Immuno-Compromised Populations
Escalating numbers of immune-compromised patients-kidney transplant recipients, individuals on biologic immunosuppressants, and long-COVID sufferers-are broadening the Kaposi's Sarcoma treatment market beyond the historical HIV cohort. Kidney transplant recipients face added complexity because standard antirejection drugs dampen tumor immunity, creating demand for regimens that balance graft protection with oncologic control. Emergent data on post-viral immune dysregulation following COVID-19 further enlarges the patient universe, prompting oncologists to adapt treatment pathways.
Wider Uptake of Liposomal Anthracyclines in First-Line Therapy
Pegylated liposomal doxorubicin has become the preferred first-line agent because it decreases cardiotoxicity while sustaining antitumor potency. Three-decade follow-up confirms durable efficacy, and multiple European approvals such as Celdoxome and Myocet have fostered competition that is nudging prices downward bmjoncology.bmj.com. Expanded formulary availability in Asia-Pacific hospitals is accelerating uptake, and U.S. payers increasingly authorize outpatient administration, trimming facility overheads.
High Toxicity Profile of Existing Chemotherapies
Cumulative doxorubicin exposure above 450 mg/m2 elevates congestive-heart-failure risk to 11%, forcing shorter regimens or cardioprotective measures that raise treatment cost. Pegylated formulations reduce exposure yet hand-foot syndrome causes dose interruptions in up to 40% of recipients, curbing real-world effectiveness. Such toxicity concerns push oncologists toward immunotherapies, but access remains uneven.
Other drivers and restraints analyzed in the detailed report include:
- Growing Access to Antiretroviral Therapy in LMICs
- Pipeline of Immune-Checkpoint Inhibitors Targeting HHV-8
- Limited Reimbursement Outside HIV-Associated Cases
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Chemotherapy anchored almost half of 2024 revenue, yet its growth has slowed as clinicians seek safer long-term strategies. Liposomal anthracyclines continue to dominate first-line use because of strong data and broad formulary coverage, keeping the Kaposi's Sarcoma treatment market firmly grounded in cytotoxic approaches. Even so, immune-checkpoint drugs, especially PD-1 inhibitors, are demonstrating durable responses in refractory disease, elevating their share in trial enrollment lists. Bioscience investors have responded by directing capital toward antibody engineering and T-cell activation platforms, suggesting a structural tilt toward immunotherapy over the forecast window.
Immunotherapy's 5.34% CAGR reflects both scientific progress and practical convenience. Subcutaneous nivolumab, cleared in December 2024, trims chair time from hours to minutes, allowing outpatient centers to treat additional patients per day. These operational efficiencies resonate with health systems pivoting from inpatient infusions. Consequently, immunotherapy's contribution to the Kaposi's Sarcoma treatment market size is expected to swell from the mid-teens today to more than one-quarter by 2030, accelerating overall revenue momentum without materially lifting patient counts.
The Kaposi Sarcoma Market is Segmented by Therapy (Chemotherapy, Immunotherapy and More), by Route of Administartaion (oral, Intravenous, Antiviral Therapy (HAART), Targeted/Precision Therapies and More), by End User (Hospital, Speciality Clinics and More) and Geography (North America, Europe, Asia-Pacific, Middle East and Africa, and South America). The Report Offers the Value (in USD Million) for the Above Segments.
Geography Analysis
North America generated 39.78% of 2024 revenue, supported by mature insurance coverage, sophisticated clinical trial infrastructure, and early approval of novel agents . Yet, cost containment policies are hardening. Medicare drug-price negotiations under the Inflation Reduction Act, while easing patient out-of-pocket burdens, cloud manufacturers' pricing power, nudging some firms to delay launches until clearer guidance emerges.
Asia-Pacific posts the leading 6.67% CAGR thanks to improved HIV outreach, upgraded regulatory regimes, and aggressive hospital build-outs. China's rising oncology budget under the 14th Five-Year Plan and India's expansion of National AIDS Control Programme facilities are catalyzing demand. However, rural-urban disparities persist: cold-chain networks struggle in remote provinces, limiting high-value biologic penetration. Innovative value-based agreements, already piloted in Singapore and South Korea, may mitigate affordability gaps as regional payers experiment with outcome-linked pricing.
Europe maintains steady, mid-single-digit growth, underpinned by compulsory insurance systems that guarantee broad access but also enforce strict cost-effectiveness thresholds. Uptake of biosimilar pegylated liposomal doxorubicin has delivered double-digit savings for national health funds, freeing resources for immune-checkpoint therapies. Post-Brexit regulatory adjustments require parallel submissions to both EMA and MHRA, adding administrative cost that smaller biotech firms sometimes struggle to absorb, subtly consolidating launch activity among larger multinationals.
List of Companies Covered in this Report:
- Bristol-Myers Squibb
- Gilead Sciences
- F-Hoffmann-La Roche
- Takeda Pharmaceuticals
- Merck
- Pfizer
- Johnson & Johnson
- Abbvie
- Celltrion
- Viatris
- Novartis
- AstraZeneca
- Eli Lilly and Company
- Sanofi
- Cipla
- Dr. Reddy's Laboratories
- Lupin
- Sun Pharmaceuticals Industries
- Hikma Pharmaceuticals
- Roche
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Rising incidence among immuno-compromised populations
- 4.2.2 Wider uptake of liposomal anthracyclines in first-line therapy
- 4.2.3 Growing access to antiretroviral therapy in LMICs
- 4.2.4 Pipeline of immune-checkpoint inhibitors targeting HHV-8
- 4.2.5 AI-driven histopathology workflows shortening diagnostic lead-time (under-reported)
- 4.2.6 Cell-based gene therapies entering early-phase trials (under-reported)
- 4.3 Market Restraints
- 4.3.1 High toxicity profile of existing chemotherapies
- 4.3.2 Scarcity of validated surrogate endpoints for rare-tumor trials
- 4.3.3 Limited reimbursement outside HIV-associated cases (under-reported)
- 4.3.4 Cold-chain gaps in endemic African regions (under-reported)
- 4.4 Value/ Supply-Chain Analysis
- 4.5 Regulatory Landscape
- 4.6 Technological Outlook
- 4.7 Porter's Five Forces
- 4.7.1 Threat of New Entrants
- 4.7.2 Bargaining Power of Suppliers
- 4.7.3 Bargaining Power of Buyers
- 4.7.4 Threat of Substitutes
- 4.7.5 Competitive Rivalry
5 Market Size & Growth Forecasts
- 5.1 By Therapy (Value)
- 5.1.1 Chemotherapy
- 5.1.2 Immunotherapy
- 5.1.3 Antiviral Therapy (HAART)
- 5.1.4 Targeted/Precision Therapies
- 5.2 By Route of Administration (Value)
- 5.2.1 Intravenous
- 5.2.2 Oral
- 5.2.3 Topical
- 5.3 By End User (Value)
- 5.3.1 Hospitals
- 5.3.2 Specialty Clinics
- 5.3.3 Ambulatory Surgical Centers
- 5.4 By Geography (Value)
- 5.4.1 North America
- 5.4.1.1 United States
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 Europe
- 5.4.2.1 Germany
- 5.4.2.2 United Kingdom
- 5.4.2.3 France
- 5.4.2.4 Italy
- 5.4.2.5 Spain
- 5.4.2.6 Rest of Europe
- 5.4.3 Asia-Pacific
- 5.4.3.1 China
- 5.4.3.2 India
- 5.4.3.3 Japan
- 5.4.3.4 South Korea
- 5.4.3.5 Australia
- 5.4.3.6 Rest of Asia-Pacific
- 5.4.4 South America
- 5.4.4.1 Brazil
- 5.4.4.2 Argentina
- 5.4.4.3 Rest of South America
- 5.4.5 Middle East and Africa
- 5.4.5.1 GCC
- 5.4.5.2 South Africa
- 5.4.5.3 Rest of Middle East and Africa
- 5.4.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company Profiles (includes Global Overview, Market-level Overview, Core Segments, Financials, Strategic Information, Market Rank/Share, Products & Services, Recent Developments)
- 6.3.1 Bristol Myers Squibb
- 6.3.2 Gilead Sciences
- 6.3.3 F-Hoffmann-La Roche
- 6.3.4 Takeda Pharmaceutical
- 6.3.5 Merck & Co.
- 6.3.6 Pfizer
- 6.3.7 Johnson & Johnson
- 6.3.8 AbbVie
- 6.3.9 Celltrion
- 6.3.10 Viatris
- 6.3.11 Novartis
- 6.3.12 AstraZeneca
- 6.3.13 Eli Lilly
- 6.3.14 Sanofi
- 6.3.15 Cipla
- 6.3.16 Dr. Reddy's Laboratories
- 6.3.17 Lupin
- 6.3.18 Sun Pharma
- 6.3.19 Hikma Pharmaceuticals
- 6.3.20 Roche Diagnostics
7 Market Opportunities & Future Outlook
- 7.1 White-space & Unmet-need Assessment




