PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1842669

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1842669
Non-vascular Stents - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
The non-vascular stents market generated USD 1.81 billion in 2025 and is forecast to expand at a 4.22% CAGR, reaching USD 2.22 billion by 2030.
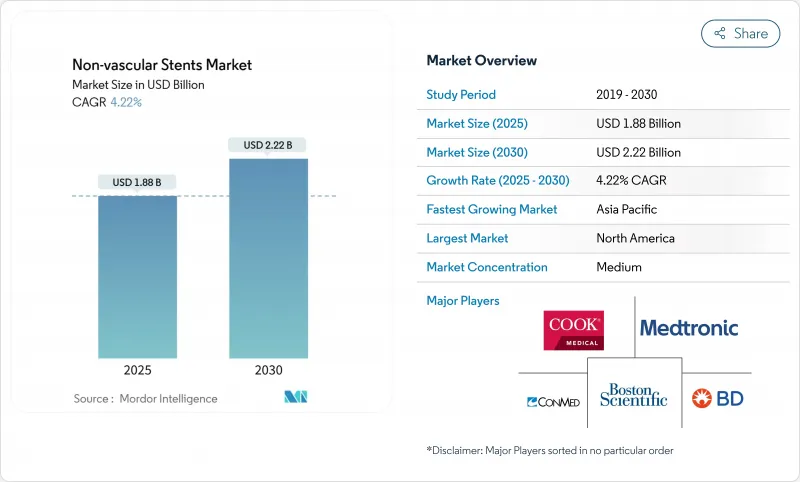
The measured growth pace reflects a maturing segment in which material science breakthroughs, especially bioresorbable polymers and patient-specific 3-D printing, complement entrenched metallic designs to meet diverse clinical demands. Manufacturers are absorbing up to 20% increases in specialty-alloy input costs, yet long-term demand resilience is anchored in a rapidly aging global population, a wider clinical shift toward minimally invasive procedures, and regulatory programs that shorten time-to-market for breakthrough devices. Competitive differentiation centers on novel coatings that curb restenosis, software-guided deployment systems that enhance procedural precision, and closer alignment between device life-cycle and sustainability mandates. Pulmonary, biliary and tracheal indications illustrate how procedure volumes continue to migrate from open surgery to endoscopic and bronchoscopic routes, reinforcing willingness to pay for advanced stent platforms.
Global Non-vascular Stents Market Trends and Insights
Growing Geriatric Population and Chronic Disease Prevalence
Population aging intersects with higher incidences of gastrointestinal, pulmonary and urological disorders, lifting procedure volumes across every therapeutic class within the non-vascular stents market. Forecasts to 2040 show colorectal, pancreatic and liver cancers remaining on an upward trajectory, creating multi-organ intervention requirements among elderly cohorts, whose tissue fragility and comorbidities demand stents with improved conformability and reduced inflammatory profiles. Growth therefore reflects not only rising absolute case numbers but also repeat procedures as patients live longer with chronic conditions.
Technological Advances in Materials and Coatings
Next-generation molybdenum-rhenium alloys combine high fatigue strength with biocompatibility, unlocking new design freedom beyond conventional nitinol Argus Media. Laser micro-patterning techniques can suppress smooth-muscle proliferation by 75% while enhancing endothelialization twofold. Drug-free collagen-functionalized platforms similarly shorten healing times without relying on anti-proliferative agents. Collectively, these innovations are widening addressable patient pools by reducing restenosis risks and metal allergy concerns.
Complications: Migration, Occlusion and Infection
Biliary stent migration appears in 8.4% of treated patients and frequently triggers cholangitis or obstruction that require urgent retrieval, adding cost and clinical burden. Rare intracardiac displacement of ureteral devices illustrates the severity spectrum, with endovascular extraction and multidisciplinary care raising hospital resource use. Despite improved anchoring designs, complication anxiety weighs on clinician decision-making, especially in regions lacking advanced retrieval tools.
Other drivers and restraints analyzed in the detailed report include:
- Rising Demand for Minimally Invasive Procedures
- 3-D-Printed Patient-Specific Stents
- Availability of Alternative Therapies
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Gastrointestinal platforms generated 42.35% of the non-vascular stents market in 2024 on the strength of entrenched protocols for esophageal, biliary and colorectal procedures. Hospitals deploy self-expandable metal designs to treat malignant obstruction where palliative decompression can avert emergency surgery and preserve quality of life. Technical success consistently exceeds 90%, while fully covered devices are entering benign esophageal strictures, broadening application scope. Pulmonary stents, although a smaller base, are expanding 7.58% per year as interventional pulmonology gains acceptance for both malignant airway obstruction and benign tracheobronchomalacia.
Clinical guidelines now position silicone and hybrid metal-silicone tubes as first-line for central lesions, but biodegradable polydioxanone alternatives have achieved 89.7% effectiveness in adult cohorts after two months, easing later removal. Custom tracheobronchial units produced through AI-enabled 3-D modeling further compress lead times compared with labor-intensive manual molding. In parallel, urological devices continue to capture physician mind-share thanks to extractable strings that cut removal pain scores from 5.23 to 0.86 and slash dwell time to 16 days, saving patients USD 146 in follow-up costs. Oral oncology stents represent another high-value niche, protecting surrounding tissue during radiotherapy and underscoring how additive manufacturing unlocks low-volume bespoke use cases.
Metallic constructions, primarily nitinol, contributed 61.54% of 2024 revenue, benefiting from decades of clinical familiarity, high radial force and kink resistance. Supply chain turbulence, however, has raised alloy costs by 20%, prompting both diversification and renewed interest in iron and magnesium bioresorbables. Second-generation magnesium scaffolds such as AMS-2.1 restore vessel strength yet complete degradation inside 720 days, answering clinician calls for temporary support that avoids permanent caging. Iron scaffolds still corrode too slowly, though surface texturing and galvanic coupling show early promise.
Polymer-based designs accelerate degradation but often rely on metallic backbones for strength; hybrid models therefore combine poly-l-lactide or poly-dioxanone sleeves with thin nitinol frameworks. Drug-eluting layers using sirolimus or paclitaxel further cut neointimal hyperplasia, supporting 8.77% growth for coated systems. Sustainability mandates are also shaping R&D, with firms testing cellulose-based delivery sheaths and recyclable tray materials to align with EU packaging rules effective in 2026.
The Non-Vascular Stents Market Report is Segmented by Product Type (Gastrointestinal Stents, Pulmonary (Airway) Stents, and More), Material Type (Metallic, Non-Metallic, and More), Design (Self-Expandable and Balloon-Expandable), End-User (Hospitals, Ambulatory Surgical Centers, and More), and Geography (North America, Europe, Asia-Pacific, and More). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America consolidated 36.44% revenue in 2024, buoyed by Medicare coverage pathways that guarantee reimbursement for breakthrough devices within six months of FDA clearance.The FDA has already granted 1,041 Breakthrough Device designations, 128 of which reached the market, turbo-charging domestic adoption of advanced polymer and AI-assisted platforms. Outpatient migration is particularly strong, with hospital outpatient departments swiftly integrating electrocautery-enhanced stents for gallbladder drainage.
Asia-Pacific is advancing at a 7.69% CAGR, the fastest worldwide, as demographic aging intersects with expanded state insurance programs. Japan remains the region's technology bellwether, importing high-precision U.S. stent systems for complex biliary and airway cases despite conservative physician adoption cycles. Regional policymakers are also courting technology transfer deals that pair Western intellectual property with local mass-production capacity, helping offset foreign-exchange exposure and supply chain volatility. Venture funding compressed in 2024 yet proprietary 3-D printed airway devices still secured regulatory approvals, signaling continued investor appetite for differentiated indications.
Europe represents a stabilizing influence with demand anchored in universal coverage schemes and early adoption of sustainability directives. Packaging regulations effective from 2026 oblige device makers to account for end-of-life recycling even in sterile environments, nudging R&D toward light-weight trays and QR-code-enabled traceability. Middle East & Africa and South America collectively hold a smaller footprint yet exhibit rising tender activity for modular endoscopy suites that support rapid deployment of stent programs in tertiary hospitals.
- Beckton Dickinson
- Boston Scientific
- Conmed
- Cook Group
- ELLA-CS s.r.o.
- Glaukos
- Hobbs Medical
- Medtronic
- Micro-tech
- W. L. Gore & Associates
- Taewoong Medical
- Olympus
- Abbott Laboratories
- Medi-Globe (EndoFlex GmbH)
- Stryker
- Merit Medical Systems
- Coloplast
- Changzhou Intl. Trade & Enterprises Cooperative Co., Ltd(CITEC)
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Growing Geriatric Population & Chronic Disease Prevalence
- 4.2.2 Technological Advancements In Materials & Coatings
- 4.2.3 Rising Demand For Minimally-Invasive Procedures
- 4.2.4 3D-Printed Patient-Specific Stents Gain Clinical Traction
- 4.2.5 Fast-Track Regulatory Pathways
- 4.2.6 Advantages Associated with Biodegradable Polymer Stents
- 4.3 Market Restraints
- 4.3.1 Complications: Migration, Occlusion & Infection
- 4.3.2 Availability Of Alternative Therapies
- 4.3.3 Supply-Chain Risk For Ni-Ti Alloys & Rare Metals
- 4.3.4 Sustainability Pressure On Single-Use Devices
- 4.4 Value / Supply-Chain Analysis
- 4.5 Regulatory Landscape
- 4.6 Technology Outlook
- 4.7 Porter's Five Forces Analysis
- 4.7.1 Bargaining Power of Suppliers
- 4.7.2 Bargaining Power of Buyers
- 4.7.3 Threat of New Entrants
- 4.7.4 Threat of Substitutes
- 4.7.5 Intensity of Competitive Rivalry
5 Market Size and Growth Forecasts (Value-USD)
- 5.1 By Product Type
- 5.1.1 Gastrointestinal Stents
- 5.1.2 Pulmonary (Airway) Stents
- 5.1.3 Urological Stents
- 5.1.4 Others
- 5.2 By Material Type
- 5.2.1 Metallic
- 5.2.2 Non-metallic
- 5.2.3 Biodegradable / Drug-eluting Coated
- 5.3 By Design
- 5.3.1 Self-expandable
- 5.3.2 Balloon-expandable
- 5.4 By End-User
- 5.4.1 Hospitals
- 5.4.2 Ambulatory Surgical Centers
- 5.4.3 Specialty Clinics
- 5.5 By Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.2 Europe
- 5.5.2.1 Germany
- 5.5.2.2 United Kingdom
- 5.5.2.3 France
- 5.5.2.4 Italy
- 5.5.2.5 Spain
- 5.5.2.6 Rest of Europe
- 5.5.3 Asia-Pacific
- 5.5.3.1 China
- 5.5.3.2 Japan
- 5.5.3.3 India
- 5.5.3.4 Australia
- 5.5.3.5 South Korea
- 5.5.3.6 Rest of Asia-Pacific
- 5.5.4 Middle East and Africa
- 5.5.4.1 GCC
- 5.5.4.2 South Africa
- 5.5.4.3 Rest of Middle East and Africa
- 5.5.5 South America
- 5.5.5.1 Brazil
- 5.5.5.2 Argentina
- 5.5.5.3 Rest of South America
- 5.5.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products and Services, and Recent Developments)
- 6.3.1 Becton, Dickinson and Company
- 6.3.2 Boston Scientific Corporation
- 6.3.3 CONMED Corporation
- 6.3.4 Cook Medical
- 6.3.5 ELLA-CS s.r.o.
- 6.3.6 Glaukos Corporation
- 6.3.7 Hobbs Medical
- 6.3.8 Medtronic plc
- 6.3.9 Micro-Tech (Nanjing) Co., Ltd.
- 6.3.10 W. L. Gore & Associates, Inc.
- 6.3.11 Taewoong Medical Co., Ltd.
- 6.3.12 Olympus Corporation
- 6.3.13 Abbott Laboratories
- 6.3.14 Medi-Globe (EndoFlex GmbH)
- 6.3.15 Stryker Corporation
- 6.3.16 Merit Medical Systems, Inc.
- 6.3.17 Coloplast A/S
- 6.3.18 Changzhou Intl. Trade & Enterprises Cooperative Co., Ltd(CITEC)
7 Market Opportunities and Future Outlook
- 7.1 White-Space and Unmet-Need Assessment




