PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1844706

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1844706
Micro Guide Catheter - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
The micro guide catheters market size reached USD 472.65 million in 2025 and is projected to climb to USD 650.01 million by 2030, advancing at a 6.58% CAGR.
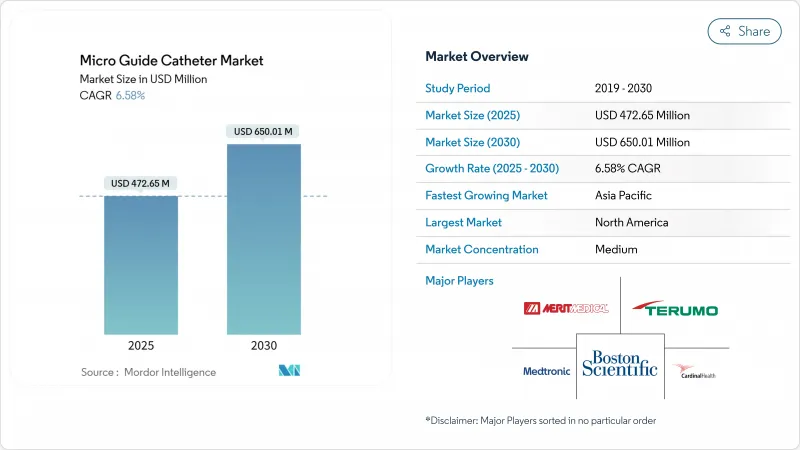
Demand is rising because aging populations need more interventional procedures, device designs now integrate steerability and pressure-sensing, and policy makers continue to shift routine angioplasty and simple neurovascular work from inpatient suites to cost-saving ambulatory settings. Cardiovascular disease now affects 127.9 million U.S. adults, spurring hospitals to expand chronic total occlusion (CTO) programs that rely on dual-lumen and locking designs able to cross heavily calcified lesions. On the neuro side, mechanical thrombectomy guidelines recommend faster access to distal territories, and computer-assisted shaping algorithms deliver 96% first-trial success, reducing procedure time and fluoroscopy. Consolidation among OEMs, such as Boston Scientific's USD 1.26 billion purchase of Silk Road Medical, adds scale for R&D and secures polymer supply, yet persistent PTFE shortages and resin plant outages hinder component availability.
Global Micro Guide Catheter Market Trends and Insights
Growing Burden Of Cardiac & Neurovascular Disorders
Cardiovascular disease imposes USD 422.3 billion in direct costs annually on the United States and continues to rise as lifestyle risk factors intersect with aging demographics. The same pattern is unfolding across Asia-Pacific, where Hong Kong spends USD 4.6 billion and Singapore USD 8.1 billion on treatment, prompting governments to subsidize interventional programs. Increasing stroke incidence means more neurologists are training in catheter skills, and multi-disciplinary stroke teams now perform a record number of thrombectomies that depend on micro guide catheter torque response and tip flexibility. Together, these factors sustain long-run demand in the micro guide catheters market.
Rising Adoption Of Minimally-Invasive Interventions
The clinical community favors less invasive solutions such as TAVR for high-risk elderly patients; self-expanding valves posted a 9.4% combined endpoint versus 10.6% for balloon-expandable platforms in small annuli. Higher volumes of structural heart cases require microcatheters with precise pressure feedback to optimize closure device positioning. Computer-guided shaping software lowers first-pass failure from 34% to 4%, trimming fluoroscopy seconds and operator fatigue. The same digital tools inform stroke systems, where speed of access dictates neurological function, thus creating a meaningful tail-wind for micro guide catheters market growth.
Shortage Of Highly-Skilled Interventional Specialists
Only one new cardiologist enters the workforce for every two that retire, limiting lab throughput in many hospitals . Burnout rates have escalated because reimbursement stagnates while case complexity rises. Early-career physicians contend with higher predicted mortality caseloads, reinforcing the need for intuitive catheters that shorten learning curves and improve confidence. Rural regions feel the shortfall most; hospitals ship patients hundreds of miles, delaying treatment and compressing demand for micro guide catheters market devices in underserved areas.
Other drivers and restraints analyzed in the detailed report include:
- Expanding Geriatric Patient Pool Worldwide
- Surge In CTO-PCI Driving Demand For Dual-/Locking Microcatheters
- Supply-Chain Volatility For High-Performance Polymers
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Over-the-wire configurations held 65.35% of revenue in 2024 as operators continue to favor their incremental support during prolonged balloon inflations and stent delivery. This category's entrenched clinical familiarity keeps it at the center of CTO protocols, yet flow-directed systems are picking up pace at a 7.57% CAGR, particularly in neurovascular circles that value atraumatic distal access. Boston Scientific's Renegade HI-FLO exemplifies design gains, posting 36.8% lower force and 7% higher flow than peers . Steerable shafts reduce wire exchanges, helping labs trim fluoroscopy and contrast.
Innovation converges on dual-lumen and tri-lumen form factors that facilitate simultaneous wire or microcoil delivery. Gore's tri-lumen design handles up to four wires, simplifying fenestrated graft placement. Pressure-sensing lumens promise integrated physiological assessment but remain niche due to calibration limits that trail dedicated wires by 0.03 units in fractional-flow reserve accuracy. As resin suppliers stabilize capacity, manufacturers aim to merge steerability, modularity, and low-profile distal shafts into next-gen lines, ensuring the micro guide catheters market continues its shift toward versatile hybrids.
The Micro Guide Catheters Market Report is Segmented by Product Type (Over-The-Wire Micro Guide Catheters, Flow-Directed Micro Guide Catheters), Application (Cardiovascular, Neurovascular, Others), End User (Hospitals & Clinics, Ambulatory Surgical Centers), and Geography (North America, Europe, Asia-Pacific, Middle East & Africa, South America). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America anchors 42.81% of 2024 revenue on the strength of comprehensive insurance coverage, high lab density, and rapid adoption of precision navigation features. U.S. operators perform more than 500,000 PCI cases annually, with 29% involving CTO techniques that elevate microcatheter pull-through rates. Canada adopts a hub-and-spoke approach where community hospitals send complex patients to academic centers, increasing national device turnover. Reimbursement for pressure-sensing catheters remains favorable, with CMS paying an extra USD 989 per use when documented in outpatient claims.
Asia-Pacific, projected at an 8.45% CAGR through 2030, will be the swing territory for incremental gains in the micro guide catheters market. China opens more than 250 cath labs each year, and its volume-based-procurement tenders push domestic firms toward differentiated niches like steerable distal tips to escape commodity price ceilings. Japan advances next as an aging society with universal coverage; its neutral reimbursement for novel neuro thrombectomy tools accelerates early adoption. Southeast Asian economies such as Vietnam log double-digit medical device growth rates, though they rely on imports for sophisticated micro guide catheters. Local clinical trial participation increased 65% between 2021 and 2024, enabling faster in-country registrations.
Europe presents stable mid-single-digit expansion fueled by Germany, France, and the UK. The EU Medical Device Regulation (MDR) lengthens approval cycles but raises perceived safety, supporting clinician confidence. Latin America's fragmented payer mix tempers volume, yet Brazil's USD 59 million vascular device market sets a foothold for premium catheters targeting private hospitals. Middle East hubs like Saudi Arabia invest in cardiac centers of excellence within Vision 2030, creating procurement contracts that often bundle imaging hardware with disposables.
- Boston Scientific
- Terumo
- Medtronic
- Asahi Intecc
- Merit Medical Systems
- Cook Group
- Cardinal Health
- Teleflex
- Integer Holdings
- Stryker
- Abbott Laboratories
- Johnson & Johnson (Cordis)
- Penumbra
- AngioDynamics
- MicroPort Scientific Corp.
- Becton Dickinson & Co.
- Lepu Medical
- Acandis
- Oscor
- iVascular S.L.U.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Growing Burden Of Cardiac & Neurovascular Disorders
- 4.2.2 Rising Adoption Of Minimally-Invasive Interventions
- 4.2.3 Expanding Geriatric Patient Pool Worldwide
- 4.2.4 Surge In CTO-PCI Driving Demand For Dual-/Locking Microcatheters
- 4.2.5 Rapid Innovation In Steerable & Pressure-Sensing Microcatheters
- 4.2.6 Expansion Of High-Volume Ambulatory Cath Labs In Ems
- 4.3 Market Restraints
- 4.3.1 Shortage Of Highly-Skilled Interventional Specialists
- 4.3.2 Product Recalls & Stringent Post-Market Surveillance
- 4.3.3 Accuracy Concerns In FFR When Using Microcatheters
- 4.3.4 Supply-Chain Volatility For High-Performance Polymers
- 4.4 Value / Supply-Chain Analysis
- 4.5 Regulatory Landscape
- 4.6 Technological Outlook
- 4.7 Porter's Five Forces Analysis
- 4.7.1 Threat of New Entrants
- 4.7.2 Bargaining Power of Buyers
- 4.7.3 Bargaining Power of Suppliers
- 4.7.4 Threat of Substitute Products
- 4.7.5 Intensity of Competitive Rivalry
5 Market Size & Growth Forecasts (Value)
- 5.1 By Product Type
- 5.1.1 Over-the-wire Micro Guide Catheters
- 5.1.2 Flow-directed Micro Guide Catheters
- 5.2 By Application
- 5.2.1 Cardiovascular
- 5.2.2 Neurovascular
- 5.2.3 Others
- 5.3 By End User
- 5.3.1 Hospitals & Clinics
- 5.3.2 Ambulatory Surgical Centers
- 5.4 By Geography
- 5.4.1 North America
- 5.4.1.1 United States
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 Europe
- 5.4.2.1 Germany
- 5.4.2.2 United Kingdom
- 5.4.2.3 France
- 5.4.2.4 Italy
- 5.4.2.5 Spain
- 5.4.2.6 Rest of Europe
- 5.4.3 Asia-Pacific
- 5.4.3.1 China
- 5.4.3.2 Japan
- 5.4.3.3 India
- 5.4.3.4 Australia
- 5.4.3.5 South Korea
- 5.4.3.6 Rest of Asia-Pacific
- 5.4.4 Middle East & Africa
- 5.4.4.1 GCC
- 5.4.4.2 South Africa
- 5.4.4.3 Rest of Middle East & Africa
- 5.4.5 South America
- 5.4.5.1 Brazil
- 5.4.5.2 Argentina
- 5.4.5.3 Rest of South America
- 5.4.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company Profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products & Services, and Recent Developments)
- 6.3.1 Boston Scientific Corporation
- 6.3.2 Terumo Corporation
- 6.3.3 Medtronic plc
- 6.3.4 Asahi Intecc Co. Ltd
- 6.3.5 Merit Medical Systems Inc.
- 6.3.6 Cook Group Incorporated
- 6.3.7 Cardinal Health Inc.
- 6.3.8 Teleflex Incorporated
- 6.3.9 Integer Holdings Corporation
- 6.3.10 Stryker Corporation
- 6.3.11 Abbott Laboratories
- 6.3.12 Johnson & Johnson (Cordis)
- 6.3.13 Penumbra Inc.
- 6.3.14 AngioDynamics Inc.
- 6.3.15 MicroPort Scientific Corp.
- 6.3.16 Becton Dickinson & Co.
- 6.3.17 Lepu Medical Technology
- 6.3.18 Acandis GmbH
- 6.3.19 Oscor Inc.
- 6.3.20 iVascular S.L.U.
7 Market Opportunities & Future Outlook
- 7.1 White-space & Unmet-Need Assessment




