PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1846281

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1846281
Joint Pain Injections - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
The Joint Pain Injections Market size is estimated at USD 5.99 billion in 2025, and is expected to reach USD 8.73 billion by 2030, at a CAGR of 7.82% during the forecast period (2025-2030).
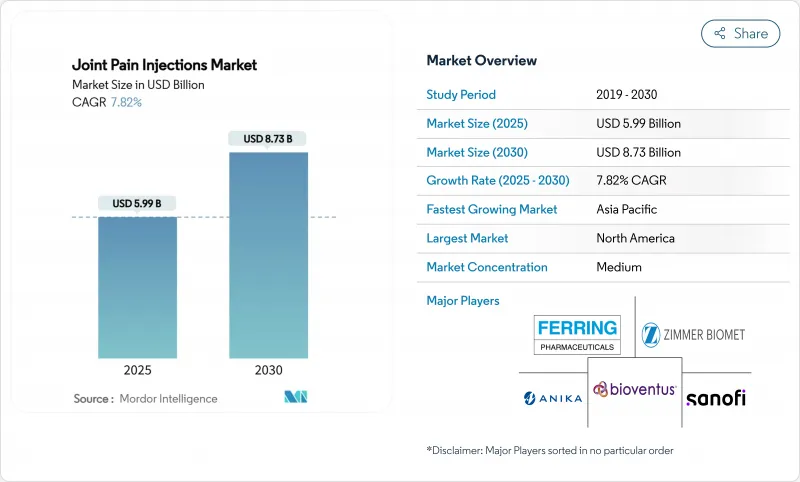
This growth aligns with a sharp rise in osteoarthritis prevalence, wider reimbursement for regenerative medicine, and steady improvements in image-guided delivery that lower complication rates. Hyaluronic acid (HA) remains the anchor therapy, yet platelet-rich plasma (PRP) and other autologous biologics are moving from experimental status to mainstream care as payers formalize coverage. Within care settings, ambulatory surgery centers (ASCs) are capturing procedure volume from hospitals thanks to 40-60% lower episode costs, while AI-enabled ultrasound drives first-pass injection accuracy above 90%. Collectively, these forces push physicians toward single-dose or three-cycle regimens that optimize chair time and delay joint-replacement surgery, improving both patient satisfaction and health-system margins.
Global Joint Pain Injections Market Trends and Insights
Increasing Osteoarthritis Incidence and Aging Population
Global osteoarthritis cases among people aged 15-64 exerting direct pressure on payers to finance minimally invasive interventions that keep working adults productive. Japan sits at the high end of prevalence with 12,610.12 cases per 100,000, an indicator of treatment demand across industrialized Asia. Post-menopausal women are driving incremental volume: nearly one-half are projected to develop osteoarthritis by 2045. These dynamics create a long-duration runway for injection therapies that defer or avoid joint replacement and maintain labor participation.
Accelerated Uptake of Single-Dose Viscosupplementation (HA)
Single-injection HA protocols cut follow-up visits while matching the efficacy of multi-injection regimens, reducing system utilization and patient travel costs. Analysis of 150,000 Korean beneficiaries showed a 44% lower total knee arthroplasty risk after single doses versus untreated cohorts, bolstering payer confidence. Industrial response is robust; BD lifted prefillable-syringe output sevenfold to satisfy anticipated demand. The operational win for providers fewer appointments has accelerated formulary placement in the United States, Germany and Japan, setting a medium-term positive arc for the joint pain injections market.
High Patient Out-of-Pocket Costs in Uninsured Segments
Coverage denials for viscosupplementation based on contested clinical value have forced some US patients to absorb the full USD 1,100-USD 1,800 per injection episode. Cigna still classifies orthopedic stem-cell therapies as "not medically necessary," creating uneven benefit structures that suppress uptake. Internationally, many emerging-market payers reimburse surgery but not biologic injections, limiting access despite clinical need. Until definitive cost-effectiveness data influence public insurance rule-making, out-of-pocket exposure will continue to curb uptake.
Other drivers and restraints analyzed in the detailed report include:
- Wider Payer Support for PRP and Regenerative Therapies
- Proliferation of Pain-Centric Ambulatory Surgery Centers (ASCs)
- Variability in Reimbursement and Procedural Coding
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
The joint pain injections market size for hyaluronic acid stood equal to 48.18% of total revenue in 2024. Long-term safety, broad payer acceptance, and rising single-dose use keep HA entrenched across knee and ankle indications. PRP revenue is forecasted for a 9.01% CAGR, gaining traction as randomized studies corroborate superior WOMAC and VAS scores at 12 months.
Combination products that merge HA with collagen tripeptides or linkers appeal to severe osteoarthritis patients and could widen choice. However, the Centers for Medicare & Medicaid Services' 2024 non-coverage determination for placental biologics narrows near-term expansion pathways. Over the forecast window, proven efficacy endpoints and payer alignment suggest PRP will chip away at HA's dominance without dislodging it entirely from first-line status in the joint pain injections market.
Single-cycle regimens represented 58.63% of market value in 2024 as physicians embraced the convenience of one-and-done dosing. Evidence now shows three PRP injections deliver statistically greater pain relief than a single dose, forming the rationale for a 12.39% CAGR through 2030 for this segment.
Five-cycle programs remain confined to severe cases because the incremental benefit plateaus after the third dose; consequently, payer willingness to reimburse beyond three sessions is waning. Manufacturers are therefore redesigning packaging-multi-chamber kits for three cycles-to remove compounding errors and shorten prep time, a move that reinforces the growth of mid-range dosing frequencies within the joint pain injections market.
The Joint Pain Injections Market Report is Segmented by Injection Type (Steroid Joint Injections, Hyaluronic Acid Injections, and More), Injection Cycle (Single-Cycle, Three-Cycle, Five-Cycle), Application (Knee & Ankle, Shoulder & Elbow, and More), End User (Hospitals, and More), and Geography (North America, Europe, Asia-Pacific, Middle East & Africa, South America). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America delivered 36.74% of 2024 sales, anchored by the United States where Medicare Advantage now reimburses PRP under specified CPT codes and employer MSK programs average USD 52 per member per month. Canada's single-payer model has begun funding HA in earlier disease stages, spurring 8% procedural growth. Mexico shows latent demand but still contends with uneven device registration timelines and out-of-pocket barriers, limiting near-term uptake.
Europe benefits from synchronized Medical Device Regulation that clarifies safety benchmarks for HA syringes. Germany and France drive regional procedure volume through robust outpatient networks, while the United Kingdom's National Health Service pilots risk-share contracts tying reimbursement to functional-outcome thresholds. Southern European countries such as Spain and Italy are scaling AI-guided ultrasound to rural clinics, boosting accessibility.
Asia-Pacific is the fastest-growing territory at 11.13% CAGR, propelled by Japan's exceptional osteoarthritis burden and well-established imaging infrastructure. China's National Medical Products Administration has slashed device approval timelines to 150 days, accelerating market entry for single-dose HA brands. Australia, India and South Korea invest heavily in ASC construction, mirroring US practice patterns and unlocking procedural capacity. Rest-of-Asia markets exhibit early-adoption behavior with government-backed tele-ultrasound programs that may compress the diffusion curve for newer injection modalities.
- Anika Therapeutics
- Bioventus
- Ferring Pharmaceuticals
- Sanofi
- Pfizer
- Eli Lilly and Company
- Flexion Therapeutics
- Teva Pharmaceutical Industries
- Zimmer Biomet
- Seikagaku
- Arthrex
- Stryker
- Sequent Scientific
- Seaspine Holdings
- Chugai Pharma
- Sequoia Medical
- Meda AB
- LG Life Science
- Terumo
- Johnson & Johnson
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Increasing Osteoarthritis Incidence and Aging Population
- 4.2.2 Accelerated Uptake of Single-Dose Viscosupplementation (HA)
- 4.2.3 Wider Payer Support for PRP and Regenerative Therapies
- 4.2.4 Proliferation of Pain-Centric Ambulatory Surgery Centers (ASCs)
- 4.2.5 Emergence of AI-Driven Ultrasound for In-Office Precision
- 4.2.6 Employer-Funded MSK Initiatives Steering Utilization of Injections
- 4.3 Market Restraints
- 4.3.1 High Patient Out-of-Pocket Costs in Uninsured Segments
- 4.3.2 Variability in Reimbursement and Procedural Coding
- 4.3.3 Clinical Concerns Surrounding Off-Label Steroid Usage
- 4.3.4 Regulatory Oversight of High-Volume Pain Clinics
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitutes
- 4.4.5 Intensity of Competitive Rivalry
5 Market Size & Growth Forecasts (Value in USD)
- 5.1 By Injection Type
- 5.1.1 Steroid Joint Injections
- 5.1.2 Hyaluronic Acid (HA) Injections
- 5.1.3 Platelet-Rich Plasma (PRP) Injections
- 5.1.4 Placental Tissue Matrix (PTM) & MSC Injections
- 5.1.5 Other Biologic / Combination Injections
- 5.2 By Injection Cycle
- 5.2.1 Single-cycle
- 5.2.2 Three-cycle
- 5.2.3 Five-cycle
- 5.3 By Application
- 5.3.1 Knee & Ankle
- 5.3.2 Shoulder & Elbow
- 5.3.3 Hip Joint
- 5.3.4 Spinal Facet & SI Joints
- 5.3.5 Other Small Joints
- 5.4 By End User
- 5.4.1 Hospitals
- 5.4.2 Ambulatory Surgical Centers (ASCs)
- 5.4.3 Orthopedic / Pain Clinics
- 5.4.4 Sports Medicine Centers
- 5.4.5 Home-Care Settings
- 5.5 By Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.2 Europe
- 5.5.2.1 Germany
- 5.5.2.2 United Kingdom
- 5.5.2.3 France
- 5.5.2.4 Italy
- 5.5.2.5 Spain
- 5.5.2.6 Rest of Europe
- 5.5.3 Asia-Pacific
- 5.5.3.1 China
- 5.5.3.2 Japan
- 5.5.3.3 India
- 5.5.3.4 Australia
- 5.5.3.5 South Korea
- 5.5.3.6 Rest of Asia-Pacific
- 5.5.4 Middle East & Africa
- 5.5.4.1 GCC
- 5.5.4.2 South Africa
- 5.5.4.3 Rest of Middle East & Africa
- 5.5.5 South America
- 5.5.5.1 Brazil
- 5.5.5.2 Argentina
- 5.5.5.3 Rest of South America
- 5.5.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company Profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products & Services, and Recent Developments)
- 6.3.1 Anika Therapeutics
- 6.3.2 Bioventus
- 6.3.3 Ferring Pharmaceuticals
- 6.3.4 Sanofi
- 6.3.5 Pfizer
- 6.3.6 Eli Lilly
- 6.3.7 Flexion Therapeutics
- 6.3.8 Teva Pharmaceuticals
- 6.3.9 Zimmer Biomet
- 6.3.10 Seikagaku Corporation
- 6.3.11 Arthrex
- 6.3.12 Stryker Corporation
- 6.3.13 Sequent Scientific
- 6.3.14 Seaspine Holdings
- 6.3.15 Chugai Pharma
- 6.3.16 Sequoia Medical
- 6.3.17 Meda AB
- 6.3.18 LG Life Science
- 6.3.19 Terumo BCT
- 6.3.20 Johnson & Johnson (DePuy Synthes)
7 Market Opportunities & Future Outlook
- 7.1 White-space & Unmet-Need Assessment




