PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1846317

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1846317
Global Pharmaceutical Continuous Manufacturing - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
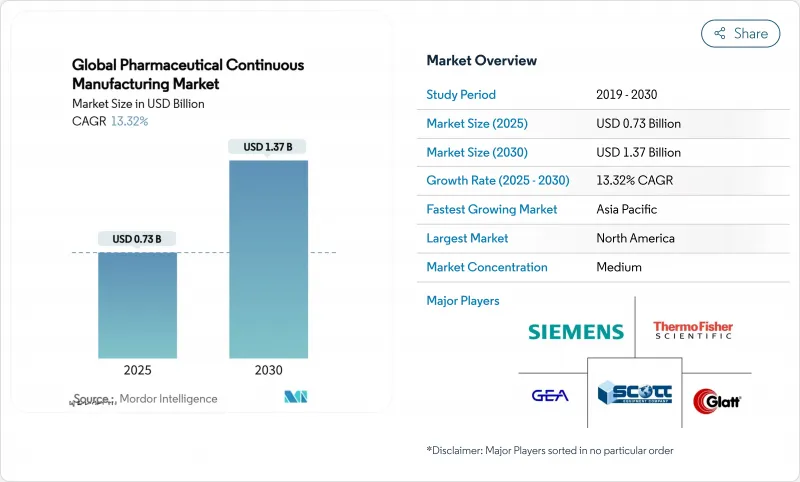
The pharmaceutical continuous manufacturing market is valued at USD 0.73 billion in 2025 and is on track to reach USD 1.37 billion by 2030, reflecting a 13.32% CAGR that outpaces most other contract-services segments.
Demand is fueled by rising biologics outsourcing, mounting cost-containment pressure, and a regulatory environment that increasingly favors experienced CDMOs. Large-scale investments in Industry 4.0-digital twins, continuous processing, and real-time release-are improving efficiency, shortening cycle times, and lowering failure rates, allowing suppliers to secure premium, multi-year contracts. Capacity constraints in high-potency APIs (HPAPIs) and advanced biologics create natural barriers to entry, while "China + 1" reshoring strategies push sponsors to diversify footprints toward North America, Europe, and cost-competitive hubs across Asia-Pacific.
Global Pharmaceutical Continuous Manufacturing Market Trends and Insights
Rising Demand for Biologics Outsourcing
Biologics manufacturing is surging as sponsors steer clear of capital-heavy facilities by engaging CDMOs with proven large-scale cell-culture, viral-vector, and fill-finish expertise. Gene therapy capacity, expanding near 30% annually, remains concentrated in a handful of service providers. FUJIFILM Diosynth Biotechnologies' 10-year, USD 3 billion supply pact with Regeneron underscores the shift to strategic partnerships anchored in volume guarantees and shared risk. The move from autologous to allogeneic platforms widens access by enabling true commercial-scale runs, while Lonza's support of more than 70 viral-vector projects highlights CDMOs' role as essential infrastructure for advanced therapies .
Cost-Containment Pressure on Pharma Innovators
Heightened pricing scrutiny and generic erosion push innovators to externalize non-core production. CDMOs absorb capex and qualification risks, offering specialized containment or aseptic suites on a pay-as-you-go basis. For early-stage biotech, external manufacturing is the only viable route to clinic, illustrated by Viking Therapeutics' USD 150 million obesity-drug deal with CordenPharma that bundles API, formulation, and finished dose capacity. Emerging milestone-based contracts further align incentives by tying payments to regulatory success.
Supply-Chain and Quality-Failure Risk
COVID-19 disruptions exposed dependencies on single-site producers, prompting dual-sourcing mandates that can splinter volumes across multiple CDMOs. Sponsors now vet suppliers for redundancy plans and real-time quality monitoring to pre-empt recalls that erode brand equity. PCI Pharma Services' pivot to dual-site fill-finish lines exemplifies the trade-off between cost efficiency and resilience . Failures carry regulatory penalties, reputational fallout, and patient-safety implications that quickly outweigh cost savings.
Other drivers and restraints analyzed in the detailed report include:
- Capacity Constraints for High-Potency APIs
- Regulatory Complexity Favouring Experienced CDMOs
- Intensifying Regulatory Audits & Warning Letters
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Finished dosage formulation generated 52.35% of 2024 revenues, underlining the premium commanded for converting APIs into patient-ready forms that comply with worldwide filing standards. Oral solids still dominate volume, yet sterile injectables, inhalables, and ophthalmics secure higher margins due to aseptic controls. The pharmaceutical continuous manufacturing market benefits as sponsors push rapid, small-batch changeovers made possible by continuous granulation and inline PAT. API manufacturing, while smaller in current share, is expected to post a 14.85% CAGR, reflecting unmet demand for HPAPI, peptide, and oligonucleotide lines capable of sub-microgram containment. Packaging and serialization are bolstered by DSCSA and EU FMD mandates that compel end-to-end traceability investments; CDMOs bundling these services capture greater wallet share and reduce supply-chain complexity for sponsors.
Growth in formulation is propelled by biopharma's focus on patient-centric dosage forms-high-concentration biologics in pre-filled syringes, nano-enabled oral suspensions, and 505(b)(2) reformulations that extend life cycles. CDMOs like Seran BioScience are commissioning spray-drying and hot-melt-extrusion lines to improve bioavailability of poorly soluble drugs. Integrated analytical and regulatory consulting now accompany most manufacturing SOWs, helping clients shorten IND-to-NDA timelines. The pharmaceutical continuous manufacturing market is leveraging continuous tablet presses and modular isolators to cut footprint, minimize operator exposure, and accelerate changeovers, positioning service providers as critical enablers of cost-efficient launches.
Small molecules retain 66.73% share yet advance steadily on the back of oncology, CNS, and anti-infective pipelines. Mature process technologies, robust supply chains, and widening adoption of continuous flow reactors sustain competitiveness. Nevertheless, biologics represent the fastest-growing value pool: monoclonal antibodies, recombinants, and vaccines rely on bioreactors, single-use systems, and high-throughput purification that few sponsors wish to maintain in-house. The pharmaceutical continuous manufacturing market size for biologics is projected to reach USD 0.64 billion by 2030, expanding at nearly 14% CAGR as commercial cell-and-gene approvals multiply.
Capital inflow is evident in Charles River Laboratories' expansion of its Memphis cell-therapy campus with nine new suites, as well as AstraZeneca's USD 300 million cell-therapy site in Rockville. CDMOs with integrative analytical, viral-vector, and GMP plasmid services enjoy premium pricing and multi-product lock-ins. Continuous downstream bioprocessing, perfusion culture, and inline viral inactivation are boosting yields while shrinking batch footprints, reinforcing biologics as the value driver within the pharmaceutical continuous manufacturing market.
The Pharmaceutical Continuous Manufacturing Market Report is Segmented by Service Type (API Manufacturing, and More), Molecule Type (Small Molecules, Large Molecules), Scale of Operation (Pre-Clinical & Clinical, Commercial), Client Type (Big Pharma, and More), and Geography (North America, Europe, Asia-Pacific, Middle East & Africa, South America). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America held 43.15% share in 2024 and sustains leadership through a dense network of innovators, seasoned regulators, and capital-rich investors. Ongoing expansions-Pfizer's Kalamazoo network and Eli Lilly's four new plants-reinforce the region's prominence. Serialisation requirements under DSCSA prompt entrenched suppliers to deploy end-to-end track-and-trace, further raising entry barriers. Long-term pacts such as FUJIFILM Diosynth Biotechnologies' USD 3 billion alliance with Regeneron signal confidence in North American expertise. The pharmaceutical continuous manufacturing market finds fertile ground here due to a reliable workforce and strong IP protection.
Asia-Pacific is the fastest-growing territory, advancing at 15.27% CAGR on account of cost-competitive labor, maturing regulatory frameworks, and large domestic patient pools. WuXi AppTec, Samsung Biologics, and emerging Indian CDMOs have achieved global scale, luring Western sponsors with hybrid price-quality propositions. "China + 1" strategies inspired by geopolitical risk and the US Biosecure Act propel investments into India, South Korea, and Southeast Asia, broadening the pharmaceutical continuous manufacturing market footprint across the region. Lotte Biologics' Songdo campus typifies the multi-billion-dollar commitments aimed at capturing surging biologics demand .
Europe maintains a robust share, anchored by harmonized EMA guidelines, skilled talent, and strong biologics clusters in Germany, Ireland, and the Nordic countries. Investments like CordenPharma's EUR 900 million peptide project and Rentschler's UK cell-and-gene facility extend the continent's capabilities. CDMOs exploit modular continuous-manufacturing lines to serve fragmented demand across 27 member states while upholding high GMP standards. Middle East & Africa and South America account for smaller portions today but see increasing local-content rules and pandemic-driven supply-security mandates that open doors for regional hubs. Collectively, regional diversification enlarges the pharmaceutical continuous manufacturing market and mitigates over-reliance on any single geography.
- Lonza Group
- Catalent
- Thermo Fisher Scientific (Patheon)
- Samsung Group
- Recipharm
- Siegfried Holding
- WuXi App Tec
- Jubilant Group
- Cambrex
- Boehringer Ingelheim BioXcellence
- Fareva
- Delpharm
- Famar
- PCI Pharma Services
- Ajinomoto Bio-Pharma Services
- Piramal Group
- Alcami
- Aenova Group
- BioVectra
- Vetter Pharma
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Rising Demand For Biologics Outsourcing
- 4.2.2 Cost-Containment Pressure On Pharma Innovators
- 4.2.3 Capacity Constraints For High-Potency Apis
- 4.2.4 Regulatory Complexity Favouring Experienced Cmos
- 4.2.5 Multi-Regional "China + 1" Reshoring Strategies
- 4.2.6 Industry 4.0 Technologies-Digital Twins, Real-Time Release, And Continuous Manufacturing
- 4.3 Market Restraints
- 4.3.1 Supply-Chain And Quality-Failure Risk
- 4.3.2 Intensifying Regulatory Audits & Warning Letters
- 4.3.3 Big-Pharma Insourcing For Strategic Mrna Platforms
- 4.3.4 Skilled-Talent Shortage At Advanced-Modality Cmos
- 4.4 Value / Supply-Chain Analysis
- 4.5 Regulatory Landscape
- 4.6 Technological Outlook
- 4.7 Porter's Five Forces Analysis
- 4.7.1 Threat of New Entrants
- 4.7.2 Bargaining Power of Buyers
- 4.7.3 Bargaining Power of Suppliers
- 4.7.4 Threat of Substitutes
- 4.7.5 Competitive Rivalry
5 Market Size & Growth Forecasts (Value)
- 5.1 By Service Type
- 5.1.1 API Manufacturing
- 5.1.2 Finished Dosage Formulation
- 5.1.2.1 Oral Solids
- 5.1.2.2 Parenterals
- 5.1.2.3 Topicals & Others
- 5.1.3 Packaging & Serialization
- 5.1.4 Other Support Services
- 5.2 By Molecule Type
- 5.2.1 Small Molecules
- 5.2.2 Large Molecules (Biologics)
- 5.2.2.1 mAbs
- 5.2.2.2 Cell & Gene Therapy
- 5.2.2.3 Vaccines & Others
- 5.3 By Scale of Operation
- 5.3.1 Pre-clinical & Clinical
- 5.3.2 Commercial
- 5.4 By Client Type
- 5.4.1 Big Pharma
- 5.4.2 Small & Mid-Sized Pharma
- 5.4.3 Biotech Companies
- 5.4.4 Generics Manufacturers
- 5.5 By Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.2 Europe
- 5.5.2.1 Germany
- 5.5.2.2 United Kingdom
- 5.5.2.3 France
- 5.5.2.4 Italy
- 5.5.2.5 Spain
- 5.5.2.6 Rest of Europe
- 5.5.3 Asia-Pacific
- 5.5.3.1 China
- 5.5.3.2 Japan
- 5.5.3.3 India
- 5.5.3.4 Australia
- 5.5.3.5 South Korea
- 5.5.3.6 Rest of Asia-Pacific
- 5.5.4 Middle East & Africa
- 5.5.4.1 GCC
- 5.5.4.2 South Africa
- 5.5.4.3 Rest of Middle East & Africa
- 5.5.5 South America
- 5.5.5.1 Brazil
- 5.5.5.2 Argentina
- 5.5.5.3 Rest of South America
- 5.5.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company Profiles {(includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products & Services, and Recent Developments)}
- 6.3.1 Lonza Group
- 6.3.2 Catalent Inc.
- 6.3.3 Thermo Fisher Scientific (Patheon)
- 6.3.4 Samsung Biologics
- 6.3.5 Recipharm AB
- 6.3.6 Siegfried Holding
- 6.3.7 WuXi AppTec
- 6.3.8 Jubilant Pharmova
- 6.3.9 Cambrex Corporation
- 6.3.10 Boehringer Ingelheim BioXcellence
- 6.3.11 Fareva
- 6.3.12 Delpharm
- 6.3.13 Famar
- 6.3.14 PCI Pharma Services
- 6.3.15 Ajinomoto Bio-Pharma Services
- 6.3.16 Piramal Pharma Solutions
- 6.3.17 Alcami Corporation
- 6.3.18 Aenova Group
- 6.3.19 BioVectra
- 6.3.20 Vetter Pharma
7 Market Opportunities & Future Outlook
- 7.1 White-space & Unmet-need Assessment




