PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1910658

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1910658
In-Vitro Diagnostics Quality Control - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2026 - 2031)
The In-Vitro Diagnostics Quality Control Market was valued at USD 1.45 billion in 2025 and estimated to grow from USD 1.51 billion in 2026 to reach USD 1.85 billion by 2031, at a CAGR of 4.15% during the forecast period (2026-2031).
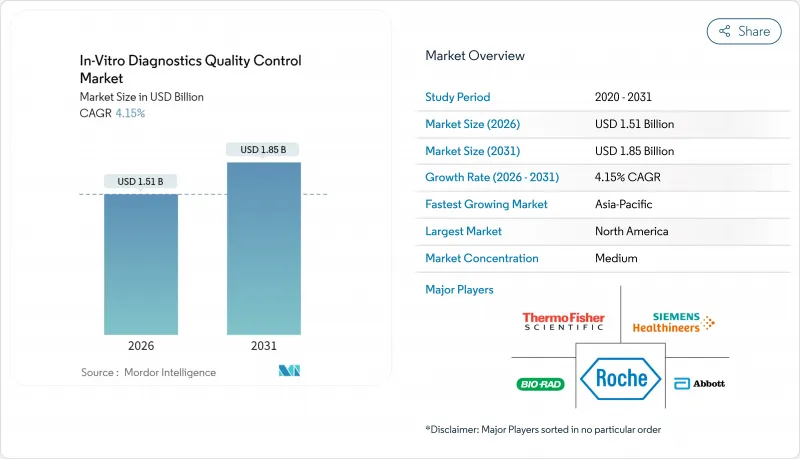
This steady pace reflects laboratories' rapid move toward real-time monitoring, wider point-of-care adoption, and stringent accreditation demands. Asia-Pacific is expanding the fastest, helped by government-funded testing networks and ISO 15189 rollouts that lift demand for third-party materials. Quality control products still dominate revenue, yet data management solutions are scaling quickly as labs seek peer benchmarking dashboards that flag errors before they reach patients. Molecular diagnostics, driven by precision-medicine protocols, is sharpening the need for synthetic controls that can verify complex gene-based assays. Market leaders are responding with integrated software-plus-material bundles that streamline compliance workflows.
Global In-Vitro Diagnostics Quality Control Market Trends and Insights
Increased Demand for Advanced Diagnostics for Sensitive Reports and Accurate Diagnosis
Molecular oncology panels and high-throughput infectious disease assays now underpin many clinical decisions, forcing labs to tighten analytical precision. Complex multiplex tests require controls that mimic patient samples and perform across varied reagent lots, driving adoption of third-party materials traceable to international standards. The World Health Organization notes that robust laboratory quality management underpins timely, reliable test outcomes. As assay complexity rises, software dashboards that trend Levy-Jennings plots in real time are becoming routine, letting supervisors intervene before deviation affects results. Vendors are bundling such analytics with controls to embed quality into daily workflows, helping labs satisfy ISO 15189 clauses on continual improvement.
Rise in Global Incidence of Infectious Diseases, Cancers and Genetic Disorders
Frequent outbreaks and an aging population with higher cancer prevalence expand molecular testing volumes, raising the stakes for accurate QC. During the COVID-19 crisis, more than 300 molecular tests gained emergency authorization, yet performance varied widely, exposing gaps in legacy QC models. The 360Dx Coronavirus Test Tracker recorded scores of new assays across the US, EU, and Asia. Developers responded with flexible, pathogen-specific controls that laboratories could calibrate quickly as variants emerged. Similar innovation is extending to respiratory panels and hereditary cancer screens, where synthetic controls stabilize workflows and curb biosafety risks.
Unfavorable Reimbursement Policies for IVD Industry
Lower payment schedules from public and private insurers are squeezing clinical laboratory margins, making it difficult to fund comprehensive quality control programs. Recent Medicare fee-schedule cuts in the United States and similar tariff reviews in Europe have reduced allowable charges for routine chemistry and immunoassay tests, yet they do not cover the incremental costs of advanced third-party controls. Many hospital networks now negotiate bundled contracts that cap test prices for multiple years, further limiting flexibility to invest in new QC platforms. Smaller independent labs are especially affected; they often postpone software upgrades and continue using minimal daily controls that meet only baseline compliance. Manufacturers report slower uptake of premium data analytics packages in markets where reimbursement ceilings remain static.
Other drivers and restraints analyzed in the detailed report include:
- Rise in the Volume of Accredited Clinical Laboratories and Adoption of Third-Party Quality Controls
- Decentralisation of Testing Driving Point-of-Care QC Demand in Emerging Economies
- Lack of Stringent Regulations for Clinical Laboratory Accreditation in Several Emerging Economies
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Quality control products generated the bulk of revenue in 2025, securing 71.32% of the in-vitro diagnostics quality control market size. They remain essential for daily assay validation, yet their growth is steady rather than spectacular. Laboratory managers are pivoting toward data management platforms that integrate middleware, peer analytics, and accreditation documentation. These platforms are forecast to post an 10.67% CAGR through 2031, reflecting a structural leap toward preventive quality models. Early adopters report shorter troubleshooting cycles and reduced proficiency-testing failures, outcomes that directly support ISO 15189 accreditation audits.
Digital tools also allow vendors to upsell subscription services such as cloud storage, mobile alerts, and predictive maintenance. The resulting recurring revenue improves manufacturers' visibility while reducing manual charting for laboratories. Because these platforms track reagent lot shifts across multiple analyzers, they lower the volume of physical controls consumed, easing supply pressures during global shortages. As awareness spreads, more stakeholders recognize that data management solutions are no longer optional add-ons but core components of a modern quality ecosystem, underpinning the in-vitro diagnostics quality control market's evolution into a service-driven model.
The in Vitro Diagnostics Quality Control Market Report is Segmented by Products and Services (Quality Control Products, Data Management Solutions, Quality Assurance Services), Application (Immunochemistry, Clinical Chemistry, Hematology, and More), End User (Hospitals, and More), and Geography (North America, Europe, Asia-Pacific, Middle East & Africa, South America). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America led with 44.73% of 2025 revenue, bolstered by CLIA's oversight of roughly 320,000 laboratory entities that must document daily QC performance. The region also hosts most top reagent makers, enabling same-day replenishment and extensive field-service support. Health systems are scaling enterprise middleware that aggregates QC data across hospital chains, raising orders for interoperable control materials and analytics subscriptions that align with cybersecurity mandates. Furthermore, reimbursement pressures are prompting laboratories to adopt patient-based real-time QC, which cuts consumables yet depends heavily on robust software algorithms.
Asia-Pacific, with an 10.89% regional CAGR, remains the standout growth engine for the in-vitro diagnostics quality control market. China's push for mutual recognition of lab results and India's expansion of national cancer screening platforms are major tailwinds. Many provincial centers in China have upgraded to ISO 15189, creating demand for third-party controls that satisfy unbiased testing criteria. Regional distributors report heightened interest in lyophilized chemistry controls that tolerate warm-chain logistics, and cloud QC portals localized in Mandarin or Hindi experience rapid adoption as they simplify accreditation traceability.
Europe presents a mature yet steadily evolving landscape. Harmonized test-result exchange across borders is stimulating investments in controls verified against commutable reference standards to ensure comparability between different analyzers. The European trend toward task-shifting sees more rapid tests executed in primary care, expanding need for compact QC vials packaged with detailed remote-training modules. Suppliers that integrate barcoded lot registration into local Laboratory Information Management Systems enjoy stronger tender wins, supporting mid-single-digit growth in a regulatory environment that prizes documented traceability.
- Abbott Laboratories
- Bio-Rad Laboratories
- Thermo Fisher Scientific
- Roche
- bioMerieux
- Siemens Healthineers
- QuidelOrtho
- Danaher
- DiaSorin
- Sysmex
- Randox Laboratories
- Technopath Clinical Diagnostics
- LGC Clinical Diagnostics (SeraCare & Maine Standards)
- Microbiologics
- ZeptoMetrix
- Qnostics Ltd.
- Fortress Diagnostics Ltd.
- Seronorm AS
- Alpha Laboratories Ltd.
- Instand e.V.
- Bio-Techne
- Sun Diagnostics LLC
- Matrical Bioscience
- Universal Biosensors Inc.
- ImmunoDNA Laboratories
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Increased Demand for Advanced Diagnostics for Sensitive Reports and Accurate Diagnosis
- 4.2.2 Rise in Global Incidence of Infectious Diseases, Cancers and Genetic Disorders
- 4.2.3 Rise in the Volume of Accredited Clinical Laboratories and Adoption of Third-Party Quality Controls
- 4.2.4 Decentralisation of Testing Driving Point-of-Care QC Demand in Emerging Economies
- 4.2.5 Technological Advancements in Molecular IVD Platforms
- 4.2.6 Global Shift Toward Regulatory Harmonization and ISO Accreditation
- 4.3 Market Restraints
- 4.3.1 Unfavorable Reimbursement Policies for IVD Industry
- 4.3.2 Lack of Stringent Regulations for Clinical Laboratory Accreditation in Several Emerging Economies
- 4.3.3 Supply Chain Disruptions Affecting QC Material Availability
- 4.3.4 Limited Digital Infrastructure in Public Sector Labs
- 4.4 Regulatory Landscape
- 4.5 Porter's Five Forces Analysis
- 4.5.1 Threat of New Entrants
- 4.5.2 Bargaining Power of Buyers
- 4.5.3 Bargaining Power of Suppliers
- 4.5.4 Threat of Substitutes
- 4.5.5 Competitive Rivalry
5 Market Size & Growth Forecasts (Value in USD)
- 5.1 By Products & Services
- 5.1.1 Quality Control Products
- 5.1.2 Data Management Solutions
- 5.1.3 Quality Assurance Services
- 5.2 By Application
- 5.2.1 Immunochemistry
- 5.2.2 Clinical Chemistry
- 5.2.3 Hematology
- 5.2.4 Molecular Diagnostics
- 5.2.5 Coagulation / Hemostasis
- 5.2.6 Microbiology & Infectious Disease
- 5.2.7 Point-of-Care Testing
- 5.3 By End User
- 5.3.1 Hospitals
- 5.3.2 Independent Clinical Laboratories
- 5.3.3 IVD Manufacturers & CROs
- 5.3.4 Academic & Research Institutes
- 5.3.5 Ambulatory & Physician Office Labs
- 5.4 By Geography
- 5.4.1 North America
- 5.4.1.1 United States
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 Europe
- 5.4.2.1 Germany
- 5.4.2.2 United Kingdom
- 5.4.2.3 France
- 5.4.2.4 Italy
- 5.4.2.5 Spain
- 5.4.2.6 Rest of Europe
- 5.4.3 Asia-Pacific
- 5.4.3.1 China
- 5.4.3.2 Japan
- 5.4.3.3 India
- 5.4.3.4 Australia
- 5.4.3.5 South Korea
- 5.4.3.6 Rest of Asia-Pacific
- 5.4.4 Middle East & Africa
- 5.4.4.1 GCC
- 5.4.4.2 South Africa
- 5.4.4.3 Rest of Middle East & Africa
- 5.4.5 South America
- 5.4.5.1 Brazil
- 5.4.5.2 Argentina
- 5.4.5.3 Rest of South America
- 5.4.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company Profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products & Services, and Recent Developments)
- 6.3.1 Abbott Laboratories
- 6.3.2 Bio-Rad Laboratories Inc.
- 6.3.3 Thermo Fisher Scientific Inc.
- 6.3.4 F. Hoffmann-La Roche AG
- 6.3.5 bioMerieux SA
- 6.3.6 Siemens Healthineers AG
- 6.3.7 QuidelOrtho Corporation
- 6.3.8 Danaher Corporation (Beckman Coulter)
- 6.3.9 DiaSorin S.p.A.
- 6.3.10 Sysmex Corporation
- 6.3.11 Randox Laboratories Ltd.
- 6.3.12 Technopath Clinical Diagnostics
- 6.3.13 LGC Clinical Diagnostics (SeraCare & Maine Standards)
- 6.3.14 Microbiologics Inc.
- 6.3.15 ZeptoMetrix Corporation
- 6.3.16 Qnostics Ltd.
- 6.3.17 Fortress Diagnostics Ltd.
- 6.3.18 Seronorm AS
- 6.3.19 Alpha Laboratories Ltd.
- 6.3.20 Instand e.V.
- 6.3.21 Bio-Techne Corporation
- 6.3.22 Sun Diagnostics LLC
- 6.3.23 Matrical Bioscience
- 6.3.24 Universal Biosensors Inc.
- 6.3.25 ImmunoDNA Laboratories
7 Market Opportunities & Future Outlook
- 7.1 White-space & Unmet-Need Assessment




