PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1848161

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1848161
Testosterone Replacement Therapy - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
The testosterone replacement therapy market size is valued at USD 2.05 billion in 2025 and is forecast to reach USD 2.51 billion by 2030, reflecting a 4.1% CAGR as rising diagnosis rates in developed countries offset pricing and access constraints in emerging economies.
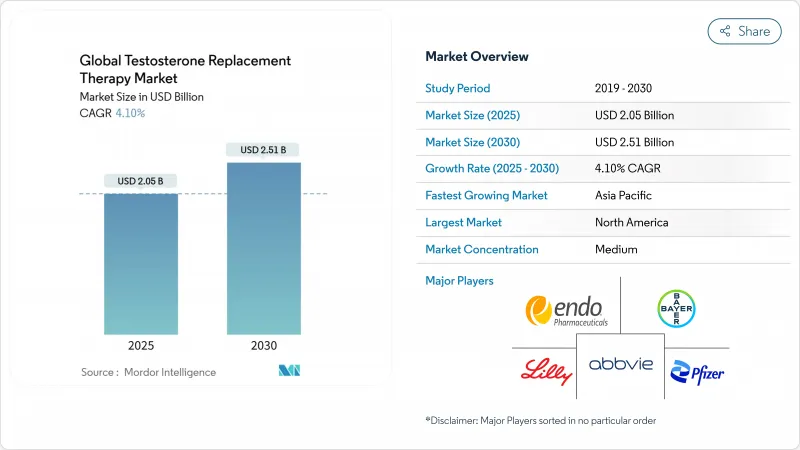
Momentum stems from the FDA's February 2025 decision to remove the cardiovascular black-box warning from testosterone labels while adding blood-pressure monitoring requirements, a move that widens prescriber confidence and patient eligibility. Growth is further reinforced by a 39% prevalence of testosterone deficiency among men older than 45 in the United States sec.gov and direct-to-consumer telehealth spending exceeding USD 400 million in 2024, indicating strong demand for convenient care models. Injectables retain market leadership, but oral formulations are advancing fastest on the back of new absorption technologies, while Asia Pacific delivers the highest regional CAGR at 5.3% through 2030. Together, these forces keep the testosterone replacement therapy market on a steady upward path despite maturing growth in North America and Western Europe.
Global Testosterone Replacement Therapy Market Trends and Insights
Growing Prevalence of Age-Related Hypogonadism in Developed Economies
Age-related hypogonadism now affects about 20% of men over 60, and metabolic comorbidities such as obesity and diabetes further enlarge the pool of candidates for therapy. The TRAVERSE trial confirmed that testosterone does not elevate major adverse cardiac event risk, easing physician concerns and encouraging earlier treatment. In countries with rapidly aging populations, demand for physiologic hormone replacement is becoming embedded in routine men's health screening. As a result, the testosterone replacement therapy market is expected to sustain long-term volume growth even as pricing pressure intensifies.
Expanding Insurance & Reimbursement Coverage for Testosterone Deficiency Therapies
Medicare now covers testosterone therapy for symptomatic hypogonadism rooted in primary or secondary gland dysfunction, provided biochemical confirmation is secured through two separate tests. Commercial insurers have broadly followed suit, shrinking patient out-of-pocket obligations and lifting prescription volumes. Clear coverage criteria have also spurred drug developers to refine diagnostic algorithms and companion testing kits. Over the medium term, expanding reimbursement breadth should help the testosterone replacement therapy market penetrate underserved demographics without undermining payer cost controls.
Stringent Regulatory Scrutiny on Cardiovascular Safety of TRT Products
Although the FDA removed the black-box warning, updated labels still demand blood-pressure monitoring and caution against off-label use for age-related hypogonadism. European regulators released a parallel statement endorsing safety for well-selected patients, yet emphasize ongoing surveillance. These guardrails deter indiscriminate prescribing and impose added compliance costs on manufacturers and clinicians, tempering near-term sales acceleration within the testosterone replacement therapy market.
Other drivers and restraints analyzed in the detailed report include:
- Advances in Long-Acting & Patient-Friendly Drug-Delivery Technologies
- Proliferation of Telehealth Platforms Streamlining TRT Access & Monitoring
- High Therapy Costs and Limited Reimbursement in Emerging Markets
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Injectables generated the largest portion of the testosterone replacement therapy market size in 2024, reaching 55.0% share on the strength of proven efficacy and affordability. The segment received a boost from Azmiro, the first prefilled testosterone cypionate syringe designed for safe in-office or at-home use. In parallel, oral capsules and soft-gels are expanding at 5.8% CAGR due to lymphatic absorption technologies that minimize hepatic risk and simplify dosing. Patients seeking discreet, pill-based regimens are gravitating toward KYZATREX, which restored normal serum levels in 96% of men during extension trials.
Topical gels and patches retain meaningful share but face hurdles around dermal transfer and variable absorption, prompting manufacturers to incorporate permeation enhancers and quick-dry solutions. Niche formats such as subdermal pellets and intranasal sprays fill specialized needs for long-lasting release or rapid peak levels but contribute limited revenue. Continued innovation across oral and subcutaneous options is expected to shift prescribing patterns without displacing core injectable volumes, preserving balanced growth within the broader testosterone replacement therapy market.
Long-acting products control 62.0% share, underscoring patient preference for less frequent dosing that maintains steady hormone levels. Partnerships like Likarda-VitalTE aim to introduce next-generation extended-release injectables that could sustain therapeutic concentrations for several weeks. Meanwhile, short-acting alternatives deliver a 5.2% CAGR as clinicians tailor flexible regimens for dose titration or temporary supplementation.
Real-world persistence data from Ontario show oral therapy with the longest median continuation at 383 days, highlighting the interplay between convenience and adherence. Development programs such as nanoTconsign, financed via an NIH grant, target uniform release across four weeks to merge the adherence benefits of monthly dosing with the pharmacokinetic profile of daily therapy. These innovations should keep duration strategies central to competitive positioning in the testosterone replacement therapy market.
The Testosterone Replacement Therapy Market Report is Segmented by Product Type (Injectables, Topicals (Gels, Patches), and More), Delivery Duration (Short-Acting Formulations and Long-Acting Formulations), Route of Administration (Intramuscular, Transdermal, and More), End User (Hospitals, and More), and Geography (North America, Europe, Asia-Pacific, and More). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America generated 48.0% of the testosterone replacement therapy market size in 2024, supported by high diagnosis rates, broad insurance coverage, and rapid adoption of novel delivery systems. The FDA's label revision is expected to lift initiation rates further by alleviating cardiovascular risk concerns, though mandatory blood-pressure monitoring adds a layer of clinical oversight. Direct-to-consumer spend surpassed USD 400 million as telehealth firms capitalized on patient demand for convenient hormone management.
Europe remains a sizeable market with heterogeneous reimbursement policies that influence country-level uptake. Acceptance has grown following release of the European Expert Panel statement affirming cardiovascular safety under proper monitoring. An aging demographic and incremental telehealth adoption continue to propel steady demand despite stricter prescribing norms compared with the United States.
Asia Pacific posts the fastest regional CAGR at 5.3%, buoyed by urbanization, rising health expenditures, and a documented 21.67% prevalence of testosterone deficiency among older Indian men. High therapy costs and limited reimbursement temper penetration, yet expanding private insurance coverage and telehealth penetration are mitigating barriers. The Middle East, Africa, and South America trail in absolute spending yet exhibit pockets of rapid growth in Gulf states and Brazil where income levels and healthcare access are improving. Telemedicine models are increasingly important in rural UK and Latin American markets where specialist density is low. These diverse regional trends collectively underpin ongoing global expansion of the testosterone replacement therapy market.
- Abbvie
- Endo International
- Pfizer
- Bayer
- Eli Lilly and Company
- Besins Healthcare
- Teva Pharmaceutical Industries
- Ferring Pharmaceuticals
- Acerus Pharmaceuticals Corp.
- Viatris
- Upsher-Smith Laboratories
- Antares Pharma
- Biote Medical
- Lupin
- Cipla
- Dr. Reddy's Laboratories
- Sun Pharmaceuticals Industries
- Sandoz Group AG
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Growing Prevalence of Age-Related Hypogonadism in Developed Economies
- 4.2.2 Expanding Insurance & Reimbursement Coverage for Testosterone Deficiency Therapies
- 4.2.3 Advances in Long-Acting & Patient-Friendly Drug-Delivery Technologies
- 4.2.4 Rising Consumer Expenditure on Male Health & Wellness Programs
- 4.2.5 Proliferation of Telehealth Platforms Streamlining TRT Access & Monitoring
- 4.2.6 Product Line Extensions & Lifecycle Management by Leading Pharma Players
- 4.3 Market Restraints
- 4.3.1 Stringent Regulatory Scrutiny on Cardiovascular Safety of TRT Products
- 4.3.2 High Therapy Costs and Limited Reimbursement in Emerging Markets
- 4.3.3 Risk of Misuse for Performance Enhancement Driving Controlled Substance Policies
- 4.3.4 Supply Disruptions and API Price Volatility Impacting Generic Manufacturers
- 4.4 Regulatory Outlook
- 4.5 Porter's Five Forces Analysis
- 4.5.1 Threat of New Entrants
- 4.5.2 Bargaining Power of Buyers
- 4.5.3 Bargaining Power of Suppliers
- 4.5.4 Threat of Substitutes
- 4.5.5 Competitive Rivalry
5 Market Size & Growth Forecasts (Value, USD)
- 5.1 By Product Type
- 5.1.1 Injectables
- 5.1.2 Topicals (Gels, Patches)
- 5.1.3 Oral Capsules / Soft-Gels
- 5.1.4 Other Product Types
- 5.2 By Delivery Duration
- 5.2.1 Short-Acting Formulations
- 5.2.2 Long-Acting Formulations
- 5.3 By Route of Administration
- 5.3.1 Intramuscular
- 5.3.2 Transdermal
- 5.3.3 Oral
- 5.3.4 Subcutaneous
- 5.3.5 Intranasal
- 5.4 By End User
- 5.4.1 Hospitals
- 5.4.2 Specialty & Urology Clinics
- 5.4.3 Other End Users
- 5.5 Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.2 Europe
- 5.5.2.1 Germany
- 5.5.2.2 United Kingdom
- 5.5.2.3 France
- 5.5.2.4 Italy
- 5.5.2.5 Spain
- 5.5.2.6 Rest of Europe
- 5.5.3 Asia-Pacific
- 5.5.3.1 China
- 5.5.3.2 Japan
- 5.5.3.3 India
- 5.5.3.4 South Korea
- 5.5.3.5 Australia
- 5.5.3.6 Rest of Asia-Pacific
- 5.5.4 Middle-East and Africa
- 5.5.4.1 GCC
- 5.5.4.2 South Africa
- 5.5.4.3 Rest of Middle East and Africa
- 5.5.5 South America
- 5.5.5.1 Brazil
- 5.5.5.2 Argentina
- 5.5.5.3 Rest of South America
- 5.5.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Strategic Moves
- 6.3 Market Share Analysis
- 6.4 Company Profiles (includes Global level Overview, Market level overview, Core Business Segments, Financials, Headcount, Key Information, Market Rank, Market Share, Products and Services, and analysis of Recent Developments)
- 6.4.1 AbbVie Inc.
- 6.4.2 Endo International PLC
- 6.4.3 Pfizer Inc.
- 6.4.4 Bayer AG
- 6.4.5 Eli Lilly & Company
- 6.4.6 Besins Healthcare
- 6.4.7 Teva Pharmaceutical Industries Ltd.
- 6.4.8 Ferring Pharmaceuticals
- 6.4.9 Acerus Pharmaceuticals Corp.
- 6.4.10 Viatris Inc. (Mylan)
- 6.4.11 Upsher-Smith Laboratories
- 6.4.12 Antares Pharma Inc.
- 6.4.13 Biote Medical
- 6.4.14 Lupin Ltd.
- 6.4.15 Cipla Ltd.
- 6.4.16 Dr. Reddy's Laboratories Ltd.
- 6.4.17 Sun Pharmaceutical Industries Ltd.
- 6.4.18 Sandoz Group AG
7 Market Opportunities & Future Outlook
- 7.1 White-Space & Unmet-Need Assessment




