PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1848165

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1848165
Homeopathy Product - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
The Homeopathy Product Market size is estimated at USD 11.12 billion in 2025, and is expected to reach USD 19.61 billion by 2030, at a CAGR of 12.02% during the forecast period (2025-2030).
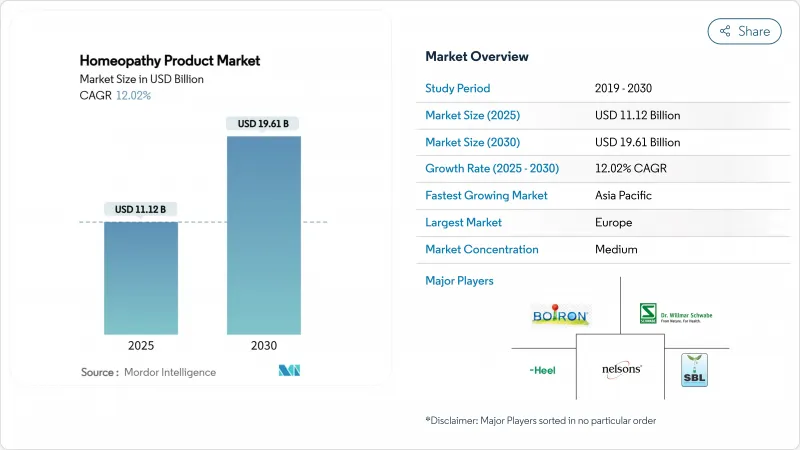
Mounting consumer distrust of conventional pharmaceuticals, wider regulatory integration in India and parts of Europe, and the proliferation of digital distribution models are the chief forces behind this expansion. Robust public healthcare debates in France and statutory health-insurance coverage in Germany further strengthen institutional adoption. Asia-Pacific sits on a steeper growth curve as India's AYUSH framework and China's large middle-class accelerate demand for natural therapies. Parallel innovation in tablets, creams, and personalized digital remedy platforms enhances product accessibility, while e-commerce enables smaller firms to reach first-time buyers without pharmacy gatekeepers. Growth momentum is tempered by supply chain risks for botanical inputs and tightening FDA guidance.
Global Homeopathy Product Market Trends and Insights
Surge in Consumer Shift Toward Natural and Holistic Therapies
Healthcare consumers increasingly prioritize natural therapeutic approaches over synthetic pharmaceuticals, driven by growing awareness of side effects and a desire for holistic wellness solutions. The uptake of complementary medicine has climbed further as millennials and Gen Z align their wellness routines with sustainable, plant-based solutions. Pediatric and chronic-care segments register particularly high repeat purchase patterns because perceived safety encourages long-term use. Strong social media advocacy and celebrity endorsements normalize homeopathic self-care, while retail chains position low-potency OTC remedies near mainstream analgesics, boosting visibility. These factors sustain baseline demand even when economic cycles dampen discretionary healthcare spending.
Rising Prevalence of Lifestyle-Linked Chronic Ailments
Chronic diseases linked to modern lifestyle factors, including diabetes, cardiovascular conditions, and stress-related disorders, drive sustained demand for complementary therapeutic approaches that address root causes rather than merely managing symptoms. Randomized trials indicate homeopathy can lower insulin resistance and relieve post-COVID-19 fatigue, outcomes that appeal to an aging population managing multiple prescriptions. Health-economic reviews show that 14 out of 21 studies found that homeopathic care produced similar or better results at an equal or lower cost than conventional treatment. As insurers seek cost control, such findings catalyze pilot reimbursement programs and foster physician referrals.
Heightened Regulatory Scrutiny & Delisting from Reimbursement Schemes
The U.S. Food and Drug Administration's revised guidance on homeopathic products creates market constraints through its "unapproved new drug" designation. This regulatory framework enables the FDA to remove homeopathic medicines from the market when necessary, limiting product availability and market expansion. Import alerts and recalls have already forced some manufacturers like Natural Ophthalmics to cease operations, demonstrating the immediate market impact of regulatory enforcement actions. European markets face similar pressures, with France and England experiencing defunding initiatives from Complementary and alternative medicine (CAM) skeptics who challenge homeopathy's integration into public health systems. Compliance costs rise, especially for small firms required to prove GMP standards and retain scientific dossiers. The threat of sudden delisting also makes pharmacies cautious about inventory levels, dampening shelf exposure.
Other drivers and restraints analyzed in the detailed report include:
- Integration of Homeopathy into National Health Systems
- Expanding Product Innovation and Distribution Channels
- Scientific Scepticism Eroding Prescriber Confidence
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Analgesic & antipyretic solutions controlled 33.21% of the homeopathy products market size in 2024, reflecting entrenched use for everyday pain and fever. Respiratory applications, however, register the highest pace at a 13.68% CAGR as consumers prioritize lung health and immune resilience after COVID-19. Short relief cycles and observable symptom relief strengthen user confidence and accelerate word-of-mouth referrals. Emerging applications in areas like diabetes management and cardiovascular health reflect expanding clinical research that validates homeopathy's role in metabolic disorder treatment, potentially opening new therapeutic categories for market expansion.
Neurology categories benefit as stress-management and sleep-support formulas gain traction among knowledge-economy workers. Dermatology leverages homeopathy's holistic branding to address chronic conditions such as eczema, where sufferers often cycle through multiple conventional therapies. Gastroenterology keeps a steady growth trajectory as research explores gut-brain interaction and microbiome modulation, areas that resonate with functional-medicine clinics. Together, these diversified indications buffer the homeopathy products market against single-segment volatility.
The Homeopathy Product Market Report is Segmented by Product Type (Tinctures, Dilutions, Tablets, Ointments & Creams, Others), Application (Analgesic & Antipyretic, Respiratory, and More), Source (Plant-Based, Animal-Based, Mineral-Based), Distribution Channel (Retail Pharmacies & Drugstores, Homeopathic Clinics, and More), and Geography (North America, Europe, and More). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
Europe commanded 32.16% of global revenues in 2024 on the back of decades-long cultural acceptance and favorable reimbursement in Germany. Growth here is slower than in emerging regions, yet product premiums remain high, preserving profit margins. France's reimbursement debate shapes investor sentiment; partial delisting would nudge patients toward OTC self-purchase rather than eliminate demand. The United Kingdom's post-Brexit regulatory independence creates opportunities for divergent homeopathy policies, though scientific skepticism within medical establishments continues challenging market expansion.
Asia-Pacific is the engine of volume expansion, moving at a 14.19% CAGR as India's AYUSH hospitals normalize doctor-prescribed remedies and China's wellness-focused middle class adopts natural therapies. Japan and Australia present developed market opportunities with sophisticated regulatory frameworks, while South Korea's growing wellness consciousness drives natural product adoption. The WHO's Traditional Medicine Strategy encourages regional governments to integrate complementary medicine into national health policies, potentially accelerating market development across diverse Asia-Pacific economies.
North America demonstrates steady growth despite regulatory headwinds. Canada's Natural Health Product framework classifies most remedies as low-risk, expediting approvals, while U.S. firms navigate the FDA's evolving stance. Mexico experiences a burgeoning private-clinic scene, and cross-border e-commerce from U.S. platforms exposes Mexican consumers to a wider assortment. The Middle East, Africa, and South America represent emerging markets with significant growth potential due to increasing healthcare awareness and expanding distribution networks. However, these regions face challenges such as economic volatility and regulatory uncertainty, which affect market development in the short term.
- A Nelson & Co Ltd.
- Ainsworths Ltd
- Allen Healthcare Co. Ltd.
- Allen Homoeo & Herbal Products Limited
- Biologische Heilmittel Heel
- Boiron
- Dr. Reckeweg & Co. GmbH
- Dr. Willmar Schwabe GmbH & Co. KG (DHU)
- Fourrts
- Hahnemann Laboratories
- Hering Pharma
- Hevert-Arzneimittel GmbH & Co. KG
- Homeocan
- Hyland's Inc.
- Medisynth Chemicals
- PEKANA Naturheilmittel GmbH
- Powell Laboratories Pvt. Ltd
- SBL Pvt Ltd
- Similasan AG
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Surge in Consumer Shift Toward Natural and Holistic Therapies
- 4.2.2 Rising Prevalence of Lifestyle-Linked Chronic Ailments
- 4.2.3 Integration of Homeopathy into National Health Systems (E.G., India, France)
- 4.2.4 Expanding Product Innovation and Distribution Channels
- 4.2.5 AI-Driven Personalised Remedy-Selection Apps
- 4.2.6 Rapid Growth of E-Commerce and D2C Fulfilment For Remedies
- 4.3 Market Restraints
- 4.3.1 Heightened Regulatory Scrutiny & Delisting from Reimbursement Schemes
- 4.3.2 Scientific Scepticism Eroding Prescriber Confidence
- 4.3.3 Shortage & Price Volatility of Alcohol and Sustainable Botanical Inputs
- 4.3.4 Social-Media Misinformation Backlash & "Post-Pandemic Evidence" Debates
- 4.4 Supply-Chain Analysis
- 4.5 Regulatory Landscape
- 4.6 Technological Outlook
- 4.7 Porter's Five Forces Analysis
- 4.7.1 Threat of New Entrants
- 4.7.2 Bargaining Power of Buyers/Consumers
- 4.7.3 Bargaining Power of Suppliers
- 4.7.4 Threat of Substitute Products
- 4.7.5 Intensity of Competitive Rivalry
5 Market Size & Growth Forecasts (Value)
- 5.1 By Product Type
- 5.1.1 Tinctures
- 5.1.2 Dilutions
- 5.1.3 Tablets
- 5.1.4 Ointments & Creams
- 5.1.5 Others
- 5.2 By Application
- 5.2.1 Analgesic & Antipyretic
- 5.2.2 Respiratory
- 5.2.3 Neurology
- 5.2.4 Dermatology
- 5.2.5 Gastroenterology
- 5.2.6 Others
- 5.3 By Source
- 5.3.1 Plant-based
- 5.3.2 Animal-based
- 5.3.3 Mineral-based
- 5.4 By Distribution Channel
- 5.4.1 Retail Pharmacies & Drugstores
- 5.4.2 Homeopathic Clinics
- 5.4.3 E-commerce & D2C
- 5.4.4 Others
- 5.5 By Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.2 Europe
- 5.5.2.1 Germany
- 5.5.2.2 United Kingdom
- 5.5.2.3 France
- 5.5.2.4 Italy
- 5.5.2.5 Spain
- 5.5.2.6 Rest of Europe
- 5.5.3 Asia-Pacific
- 5.5.3.1 China
- 5.5.3.2 Japan
- 5.5.3.3 India
- 5.5.3.4 Australia
- 5.5.3.5 South Korea
- 5.5.3.6 Rest of Asia-Pacific
- 5.5.4 Middle East and Africa
- 5.5.4.1 GCC
- 5.5.4.2 South Africa
- 5.5.4.3 Rest of Middle East and Africa
- 5.5.5 South America
- 5.5.5.1 Brazil
- 5.5.5.2 Argentina
- 5.5.5.3 Rest of South America
- 5.5.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company Profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products & Services, and Recent Developments)
- 6.3.1 A Nelson & Co Ltd.
- 6.3.2 Ainsworths Ltd
- 6.3.3 Allen Healthcare Co. Ltd.
- 6.3.4 Allen Homoeo & Herbal Products Limited
- 6.3.5 Biologische Heilmittel Heel GmbH
- 6.3.6 Boiron
- 6.3.7 Dr. Reckeweg & Co. GmbH
- 6.3.8 Dr. Willmar Schwabe GmbH & Co. KG (DHU)
- 6.3.9 Fourrts
- 6.3.10 Hahnemann Laboratories
- 6.3.11 Hering Pharma
- 6.3.12 Hevert-Arzneimittel GmbH & Co. KG
- 6.3.13 Homeocan
- 6.3.14 Hyland's Inc.
- 6.3.15 Medisynth Chemicals
- 6.3.16 PEKANA Naturheilmittel GmbH
- 6.3.17 Powell Laboratories Pvt. Ltd
- 6.3.18 SBL Pvt Ltd
- 6.3.19 Similasan AG
7 Market Opportunities & Future Outlook
- 7.1 White-space & Unmet-need Assessment




