PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1848311

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1848311
Electrophoresis Reagents - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
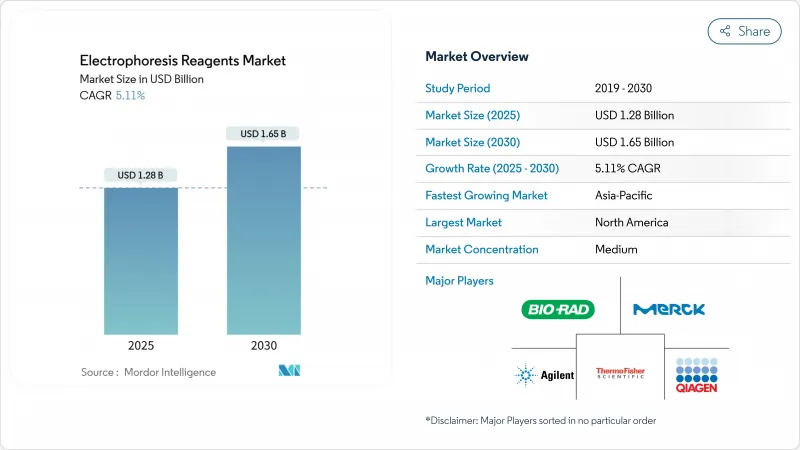
The electrophoresis reagents market size is valued at USD 1.28 billion in 2025 and is forecast to reach USD 1.65 billion by 2030, advancing at a 5.11% CAGR.
Demand is now driven by long-term shifts toward precision medicine, stricter regulatory oversight, and sustained federal research funding. The United States Food and Drug Administration's May 2024 laboratory-developed-test rule introduced a USD 566 million-USD 3.56 billion compliance burden over two decades, elevating quality thresholds that favor established reagent suppliers fda.gov. Asia-Pacific is poised to rebalance global growth as regional research hubs expand despite recent venture-financing softness. Rising adoption of greener stains, integration of AI-enabled gel analytics, and automation-heavy capillary systems further reinforce structural demand drivers for the electrophoresis reagents market.
Global Electrophoresis Reagents Market Trends and Insights
Increasing Funding for Genomic & Proteomic Research
The National Institutes of Health allocated USD 50.3 million in 2024 to its Multi-Omics for Health and Disease Consortium, signaling a sustained preference for integrated analytical platforms that require electrophoresis reagents for reliable biomarker validation. Expanded public funding compels suppliers to offer comprehensive, workflow-compatible product suites instead of stand-alone consumables. Private-sector co-investment magnifies these tailwinds, as pharmaceutical sponsors leverage public infrastructure for drug-discovery programs. Computational genomics now consumes 30% of the National Human Genome Research Institute's FY2023 budget, underscoring how data-intensive research favors high-throughput electrophoresis systems capable of generating reproducible quantitative outputs for algorithmic pipelines. Together, these funding models lengthen demand visibility across the electrophoresis reagents market by anchoring purchases to multiyear research grants.
Rising Prevalence of Chronic Diseases
The global shift toward aging populations elevates diagnostic complexity, favoring high-resolution separation techniques that can detect subtle proteomic variations in early disease states. Capillary electrophoresis supports charge-variant analysis of therapeutic proteins, a critical requirement in regulatory submissions for biopharmaceuticals. In oncology, electrophoretic separation of circulating tumor DNA enables minimally invasive liquid-biopsy assays that replace tissue-based diagnostics, thereby increasing test frequency per patient episode. Preventive-care models in developed markets convert one-off diagnostic events into recurring reagent demand, translating epidemiological trends directly into higher reagent volumes for the electrophoresis reagents market.
Time-consuming workflows & manual gel preparation
Casting polyacrylamide gels, loading samples, and post-run staining can require up to 8 hours, making labor the most significant variable cost in high-throughput environments. Bio-Rad's Stain-Free gels remove the staining step, but premium pricing and new-equipment needs deter adoption in budget-constrained labs. Global shortages of skilled technicians intensify workflow bottlenecks, prompting procurement teams to scrutinize total-cost-of-ownership and consider alternative technologies.
Other drivers and restraints analyzed in the detailed report include:
- Growing adoption of personalized medicine
- Lab-on-a-chip reagent kits for point-of-care molecular testing
- Availability of alternative separation technologies
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Gels accounted for 43.56% of the electrophoresis reagents market in 2024, underpinning virtually every separation workflow across DNA, RNA, and protein applications. Agarose remains a staple for nucleic-acid analysis thanks to low cost and straightforward casting, whereas polyacrylamide supports high-resolution protein work. Dye sales, though smaller, are set to climb at a 7.88% CAGR as ethidium-bromide replacements gain regulatory traction, reshaping the electrophoresis reagents market. Suppliers are differentiating gels through throughput-oriented formats; a 96-well polyacrylamide prototype published in 2024 demonstrated simultaneous horizontal electrophoresis with transfer compatibility, hinting at future multi-sample mainstream offerings. Buffer systems such as Tris/Acetate/EDTA remain entrenched industry standards because performance and cost stability outweigh incremental gains from proprietary modifications. Concurrently, newer dye platforms like Biotium's One-Step Lumitein series integrate staining directly into gel matrices, removing wash steps and shortening protocols-a direct response to labor-shortage challenges.
The Electrophoresis Reagents Market Report is Segmented by Product (Gel, Dyes, Buffers, and Other Products), Technique (Gel Electrophoresis and Capillary Electrophoresis), End User (Academic & Research Institutions, Pharmaceutical & Biotechnology Companies, and More), Geography (North America, Europe, Asia-Pacific, The Middle East and Africa, and South America). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America generated 40.11% of 2024 revenue for the electrophoresis reagents market, anchored by robust NIH funding and a dense pharmaceutical manufacturing base. The USD 15.4 million NIH-NSF RNA research program exemplifies the public-funding mechanism that sustains reagent consumption irrespective of macroeconomic volatility. Thermo Fisher's USD 2 billion domestic investment through 2029 underscores supplier commitment to on-shore manufacturing and R&D capacity expansion in anticipation of tax credits and reshoring incentives. Europe remains a mature yet regulation-intensive region; the Corporate Sustainability Due Diligence Directive accelerates substitution of hazardous chemicals, driving demand for greener formulations. Carl Roth's SOLVAGREEN line of recycled solvents and bioethanol illustrates how European vendors align portfolios with regulatory commitments.
Asia-Pacific is the fastest-growing sub-market with a 6.45% projected CAGR to 2030, fueled by rising government life-science budgets and expanding biopharmaceutical capacity despite a 22% venture-finance decline since 2021. China's domestic-equipment subsidies and India's production-linked incentives further tilt capital spending toward regional suppliers, though intellectual-property and supply-chain security concerns encourage multinationals to pursue joint ventures or local manufacturing branches. QIAGEN's establishment of a Riyadh hub and its memorandum with the Saudi Ministry of Health reveal how Middle East governments leverage strategic partnerships to build molecular-diagnostics ecosystems. Africa and South America remain smaller contributors; targeted donor and government health programs create episodic reagent spikes rather than smooth growth trajectories.
- Agilent Technologies
- Analytik Jena GmbH
- BioAtlas
- Bio-Rad Laboratories
- Cleaver Scientific Ltd.
- Cytiva
- Greiner Bio-One GmbH
- Helena Laboratories Corp.
- Hoefer Inc. (Harvard Bioscience)
- Labnet International Inc.
- Lonza Group
- Merck KGaA / Sigma-Aldrich
- New England Biolabs
- NIPPON Genetics Co., Ltd.
- PerkinElmer
- Promega
- QIAGEN
- Randox Laboratories
- Sebia
- SERVA Electrophoresis
- Takara Bio
- Thermo Fisher Scientific
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Increasing Funding for Genomic & Proteomic Research
- 4.2.2 Rising Prevalence of Chronic Diseases
- 4.2.3 Technological Advances in High-Throughput Electrophoresis
- 4.2.4 Growing Adoption of Personalized Medicine
- 4.2.5 Lab-On-A-Chip Reagent Kits for Point-Of-Care Molecular Testing
- 4.2.6 Shift Toward Greener, Non-Toxic Dyes & Buffers (ESG-Driven)
- 4.3 Market Restraints
- 4.3.1 Time-Consuming Workflows & Manual Gel Preparation
- 4.3.2 Availability of Alternative Separation Technologies
- 4.3.3 Acrylamide Feedstock Shortages Inflating Reagent Costs
- 4.3.4 Strict Disposal Rules for Ethidium-Bromide-Based Stains
- 4.4 Regulatory Landscape
- 4.5 Porter's Five Forces Analysis
- 4.5.1 Threat of New Entrants
- 4.5.2 Bargaining Power of Buyers
- 4.5.3 Bargaining Power of Suppliers
- 4.5.4 Threat of Substitute Products
- 4.5.5 Intensity of Competitive Rivalry
5 Market Size & Growth Forecasts (Value, USD)
- 5.1 By Product
- 5.1.1 Gels
- 5.1.1.1 Agarose Gels
- 5.1.1.2 Polyacrylamide Gels
- 5.1.1.3 Starch Gels
- 5.1.2 Dyes
- 5.1.2.1 Ethidium Bromide (EtBr)
- 5.1.2.2 Bromophenol Blue
- 5.1.2.3 SYBR Dyes
- 5.1.2.4 Other Dyes
- 5.1.3 Buffers
- 5.1.3.1 Tris/Acetate/EDTA
- 5.1.3.2 Tris/Borate/EDTA
- 5.1.3.3 Other Buffers
- 5.1.4 Other Products
- 5.1.1 Gels
- 5.2 By Technique
- 5.2.1 Gel Electrophoresis
- 5.2.2 Capillary Electrophoresis
- 5.3 By End User
- 5.3.1 Academic & Research Institutions
- 5.3.2 Pharmaceutical & Biotechnology Companies
- 5.3.3 Clinical & Diagnostic Laboratories
- 5.3.4 Other End Users
- 5.4 Geography
- 5.4.1 North America
- 5.4.1.1 United States
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 Europe
- 5.4.2.1 Germany
- 5.4.2.2 United Kingdom
- 5.4.2.3 France
- 5.4.2.4 Italy
- 5.4.2.5 Spain
- 5.4.2.6 Rest of Europe
- 5.4.3 Asia-Pacific
- 5.4.3.1 China
- 5.4.3.2 Japan
- 5.4.3.3 India
- 5.4.3.4 Australia
- 5.4.3.5 South Korea
- 5.4.3.6 Rest of Asia-Pacific
- 5.4.4 Middle East & Africa
- 5.4.4.1 GCC
- 5.4.4.2 South Africa
- 5.4.4.3 Rest of Middle East & Africa
- 5.4.5 South America
- 5.4.5.1 Brazil
- 5.4.5.2 Argentina
- 5.4.5.3 Rest of South America
- 5.4.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company Profiles (includes Global level Overview, Market level overview, Core Business Segments, Financials, Headcount, Key Information, Market Rank, Market Share, Products and Services, and analysis of Recent Developments)
- 6.3.1 Agilent Technologies Inc.
- 6.3.2 Analytik Jena GmbH
- 6.3.3 BioAtlas
- 6.3.4 Bio-Rad Laboratories Inc.
- 6.3.5 Cleaver Scientific Ltd.
- 6.3.6 Cytiva (Danaher)
- 6.3.7 Greiner Bio-One GmbH
- 6.3.8 Helena Laboratories Corp.
- 6.3.9 Hoefer Inc. (Harvard Bioscience)
- 6.3.10 Labnet International Inc.
- 6.3.11 Lonza Group AG
- 6.3.12 Merck KGaA / Sigma-Aldrich
- 6.3.13 New England Biolabs Inc.
- 6.3.14 NIPPON Genetics Co., Ltd.
- 6.3.15 PerkinElmer Inc.
- 6.3.16 Promega Corporation
- 6.3.17 Qiagen N.V.
- 6.3.18 Randox Laboratories Ltd.
- 6.3.19 Sebia Group
- 6.3.20 SERVA Electrophoresis GmbH
- 6.3.21 Takara Bio Inc.
- 6.3.22 Thermo Fisher Scientific Inc.
7 Market Opportunities & Future Outlook
- 7.1 White-space & Unmet-Need Assessment




